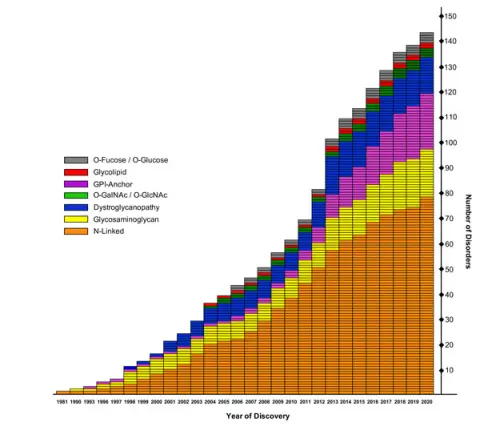
- Watch | Our inspiration for CDG research Scientists in the Hudson Freeze lab talk about their inspiration for doing Congenital Glycosylation Disorder (CDG) research
- Watch | Para-equestrian with rare disorder meets Olympian in San Diego For 20-year-old Morgan Hill, it’s a dream come true – the chance to meet an Olympic medalist and lifelong hero
- Watch | “Miracle:” Uridine at 2 days and 5.5 months Uridine at 2 days and 5.5 months
Scientist Position: Member
Emerling received her B.A. from the University of California Santa Cruz and her PhD in molecular and cellular biology from Northwestern University. Emerling did her postdoctoral training at Harvard Medical School. She then became an Instructor of Cancer Biology in Medicine at Weill Cornell Medical College in New York City, where she continued her research on lipid kinase signaling and cancer metabolism. In August 2016, Brooke joined the faculty at Sanford Burnham Prebys Medical Discovery Institute as an Assistant Professor in the Cancer Metabolism and Signaling Networks Program.
Funding Awards and Collaborative Grants
Breast Cancer Research Foundation – AACR Career Development Award for Translational Breast Cancer Research
Mary Kay Foundation Innovative Translational Grant Award
Department of Defense Breast Research Program Breakthrough Award
Honors and Recognition
2014: NextGen Star – AACR Early-Career Speaker Award
2013-2016: Mastercard Ajay Banga Scientist Award
2013: AACR – Aflac Travel Fellowship Award
Related Disease
Breast Cancer, Cancer
Phenomena or Processes
Cancer Biology, Cancer Metabolism, Cell Signaling, Metabolic Processes, Signal Transduction
Research in the Emerling Lab is focused on understanding key signaling and metabolic pathways involved in the regulation of cellular function under pathological conditions such as cancer. Our research program centers around dissecting the roles of the family of non-canonical phosphatidylinositol kinases, called the phosphatidylinositol-5-phosphate 4-kinases (PI5P4Ks), in cancer metabolism using a multi-disciplinary approach integrating human, mouse, and worm models. Currently, a major research project in the Emerling Lab is determining the role of the PI5P4Ks in p53 mutant cancers, especially the triple-negative breast cancer subgroup where targeted therapies have not been effective.
 Oct 21, 2025
Oct 21, 2025Sanford Burnham Prebys hosts inaugural event in the Women in Science Lecture Series
Oct 21, 2025The series highlights the groundbreaking work and unique perspectives of women leaders in the biomedical sciences.
 Sep 11, 2025
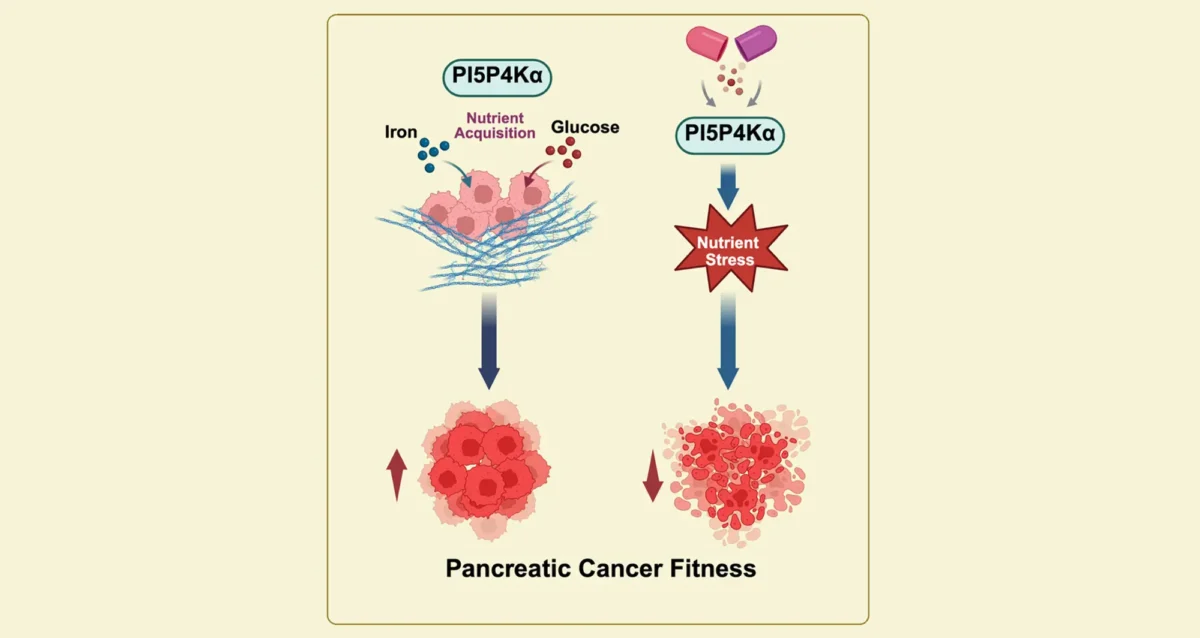
Sep 11, 2025Starving Cancer Cells of Essential Sugar and Iron
Sep 11, 2025Findings from Gurpreet Kaur Arora, Brooke Emerling, and Cosimo Commisso, identify key enzyme that pancreatic cancer cells need to obtain…
 Feb 12, 2025
Feb 12, 2025Curebound awards two grants to Sanford Burnham Prebys scientists
Feb 12, 2025The San Diego-based philanthropic organization has awarded $43 million in cancer research to date.
 Oct 18, 2024
Oct 18, 2024Two Sanford Burnham Prebys scientists selected for American Cancer Society postdoctoral fellowships
Oct 18, 2024The funds will support Alicia Llorente Lope and Ambroise Manceau who study breast and pancreatic cancer.
 May 28, 2024
May 28, 2024Overlooked lipid connected to ancient cellular pathway with links to cancer
May 28, 2024Scientists at Sanford Burnham Prebys and Vanderbilt University uncover a new role for a neglected lipid that may play a…
 Feb 3, 2023
Feb 3, 2023New treatment approach for prostate cancer could stop resistance in its tracks
Feb 3, 2023By inhibiting one enzyme, scientists from Sanford Burnham Prebys can kill prostate cancer cells when other treatments can’t For the
Previous to coming to Sanford Burnham Prebys, Dhar was an assistant professor in the Department of Medicine, Division of Gastroenterology at UC San Diego. He continues to be an adjunct faculty member.
He received his PhD in cancer gene therapy from Saint Louis University School of Medicine and his Master of Science degree in microbiology/biochemistry/molecular biology at the University of Calcutta.
Dbanjan Dhar looks at how diseases develop, specifically liver cancer.
He focuses on how lifestyle factors, such as high-calorie diets, excessive alcohol consumption and minimal exercise — along with genetic predispositions — can lead to problematic changes in the liver, heart and kidneys. Specifically in the liver, how growing deposits of fat in the tissue can lead over time to chronic inflammation and the accumulation of thickened scar tissue, a condition known as metabolic-associated steatohepatitis (MASH).
MASH is the second-leading cause of liver transplantation and a major risk factor for liver cancer.
“The liver is not an isolated organ, as it is constantly communicating with all these different tissues. “For example, there might be certain signals from the liver going into the bloodstream that may be affecting the cardiovascular system, and we just don’t have the full picture yet.”
By studying the conversation among the liver, the immune system, heart and kidneys, Dhar hopes to discover signals that could be used to detect MASH and liver cancer much earlier, when they’re easier to treat.
 Oct 27, 2025
Oct 27, 2025A Conversation About Aging and Metabolic Disorders at Sanford Burnham Prebys
Oct 27, 2025Event recording now available for panel discussion with scientists held on October 14, 2025.
 Jan 28, 2025
Jan 28, 2025A monster, MASH
Jan 28, 2025Scientists show how the advanced form of fatty liver disease has monstrous effects on liver cancer risk.
 Jan 17, 2025
Jan 17, 2025Bile may be key to immunotherapy effectiveness in liver cancer
Jan 17, 2025Understanding the crucial ingredient in bile may unlock the potential of treatments that boost patients’ immune systems.
 Aug 22, 2024
Aug 22, 2024Macrophage mix helps determine rate and fate of fatty liver disease
Aug 22, 2024The white blood cells’ typical role is to promote inflammation and stimulate the immune response, but researchers say some actually…
 Apr 17, 2024
Apr 17, 2024Debanjan Dhar looks at links among liver cancer, heart health and kidney function
Apr 17, 2024New Sanford Burnham Prebys scientist listens to the constant communication among these organs to find treatments that benefit all three
Dr. Ani Deshpande’s most recent position was as an Instructor with Dr. Scott Armstrong at the Memorial Sloan Kettering Cancer Center and the Children’s Hospital Boston/Harvard Medical School. He completed his PhD in Human Biology from the Ludwig Maximillians University in Munich. Dr. Deshpande’s research revolves around studying difficult-to-cure leukemias through the use of mouse models, genomic and epigenomic studies.
Funding Awards and Collaborative Grants
Ongoing Research Support R00 phase (number in process) Deshpande (PI) 10/0/14-present NIH/NCI – K99/R00 Howard Temin Pathway to Independence Award Role: PI (75% effort) Completed Research Support K99 CA154880 Deshpande (PI) 07/15/11-09/30/14 NIH/NCI – K99/R00 Howard Temin Pathway to Independence Award Role: Post-doctoral fellow/PI
Honors and Recognition
2014: American Society of Hematology Scholar Award (ASH Junior Faculty Scholar Award)
2013: Alex’s Lemonade Stand Foundation Travel Award for the FASEB Hematological Malignancies Meeting in Vermont, VA
2013: Abstract Achievement Award, American Society of Hematology Annual Meeting, New Orleans
2012: Abstract Achievement Award, American Society of Hematology Annual Meeting, Atlanta
2008: ASH Travel Award: 50th Annual Meeting, American Society of Hematology (ASH) San Diego
2008: The New York Stem Cell Foundation (NYSCF) Travel Grant, International Society of Stem Cell Research (ISSCR), Philadelphia
2007: The George Brecher New Investigator Award (postdoctoral) of the International Society of Experimental Hematology, Hamburg, Germany
2007: Doctoral prize of the German Society of Hematology and Oncology (Annual prize for the best doctoral thesis in hematology-oncology in Germany)
2007: The Doctoral Prize of the Helmholtz Centre, Munich for the Best Doctoral Thesis 2006, Munich, Germany
2007: Best Poster Award (2nd Prize): 36th Annual Scientific Meeting, International Society for Experimental Hematology (ISEH) Hamburg
2007: Travel Award: 36th Annual Scientific Meeting, International Society for Experimental Hematology (ISEH) Hamburg
2006: Summa Cum Laude Ludwigs Maximililans University, Munich, Germany
2005: ISEH Travel Grant: 34th Annual Scientific Meeting, International Society for Experimental Hematology (ISEH) Glasgow
2004: ISEH Travel Grant: 33rd Annual Scientific Meeting, International Society for Experimental Hematology (ISEH) New Orleans
2003: ASH Travel Award: 45th Annual Meeting, American Society of Hematology (ASH) San Diego
Related Disease
Cancer, Leukemia/Lymphoma
One of the core interests of the lab is to understand regulation of key developmental processes that are important for normal stem cells and are frequently misregulated in human cancer. We are currently investigating genetic and epigenetic abnormalities in Acute Myeloid Leukemia (AML) using mouse models, gene targeting and genome-scale approaches. Our overarching goal is to uncover therapeutic nodes of intervention in human leukemia.
Available Positions in the Deshpande Lab
We are seeking exceptional candidates with broad interests/expertise in cancer epigenetics and stem cell biology at the level of postdoctoral fellows, graduate students and undergraduates.
- Graduate students
Applicants interested in graduate positions should send their applications to the Sanford Burnham Prebys Medical Discovery Institute Graduate School for Biomedical Sciences. The application period opens in the fall. - Undergraduate students
Please contact Dr. Deshpande via email adeshpande@sbpdiscovery.org.
Ani Deshpande’s Research Report
Normal and malignant stem cells
One of the core interests of the lab is to investigate the role of chromatin regulators in benign and malignant hematopoiesis. Several attributes of normal stem cells such as the ability to self-renew are co-opted or reactivated by cancer cells on their way to malignant transformation. We are interested in characterizing the molecular determinants of “stemness” using hematopoietic stem cells as a model and in identifying ways and means by which these stem-cell associated pathways are usurped for oncogenesis.
The leukemia epigenome
Abnormal epigenetic changes have emerged as important mediators of oncogenesis. Genomic investigations of human cancer have uncovered mutations in writers, erasers and readers of the histone code. The goal of our laboratory is to connect basic mechanisms of chromatin regulation to diseased states with a focus on Acute Myeloid Leukemia (AML). Ultimately, we are interested in the rational design of screens to develop therapeutics targeting dysregulated epigenetic mechanisms in AML.
Chromosomal translocations in cancer
Ever since the discovery of the Philadelphia chromosome in 1960, a number of different chromosomal translocations have been identified in human cancer that result in the formation of potent fusion oncogenes, abnormal activation of latent proto-oncogenes, or other oncogenic events. We are very interested in investigating the impact of chromosomal translocations on the development of human cancer. Moreover, we are also interested in understanding the propensity of certain genomic loci for recurrent involvement in oncogenic chromosomal translocation events in the context of human myeloid malignancies.
 Sep 24, 2025
Sep 24, 2025How a Holocaust survivor transformed blood sugar testing
Sep 24, 2025New podcast discusses how a scientist saved from a World War II concentration camp revolutionized diabetes care.
 Sep 8, 2025
Sep 8, 2025Sizing up a weakness in synovial sarcoma’s genes
Sep 8, 2025Study shows that studying cancer’s genetic vulnerabilities can lead to new potential targeted therapies for patients.
 Aug 19, 2025
Aug 19, 2025Seeing how sugar reshapes our blood
Aug 19, 2025Scientists and podcasters tackle how a scientist in Iran overcame great odds and forever changed diabetes diagnoses.
 Jul 16, 2025
Jul 16, 2025Ani Deshpande promoted to professor in the Cancer Genome and Epigenetics Program at Sanford Burnham Prebys
Jul 16, 2025Deshpande will continue studying how blood cancers sabotage stem cells’ special features to grow and spread.
 May 27, 2025
May 27, 2025Scientists and podcasters
May 27, 2025Sanford Burnham Prebys scientists bring dramatic stories of scientific achievement to life.
 Oct 4, 2022
Oct 4, 2022How community collaboration shapes leukemia research at Sanford Burnham Prebys
Oct 4, 2022Since 2020, Todd and Rena Johnson, co-founders of the Luke Tatsu Johnson Foundation (LTJF), have helped fund the research of…
Dr. D’Angelo earned his BS and MS degrees in chemistry at the University of Cordoba, Argentina, and his PhD in biochemistry and molecular biology from University of Buenos Aires. He then trained as a postdoctoral fellow in cell biology at The Salk and the Scripps Research Institute in San Diego. In 2011, Dr. D’Angelo was appointed as an Assistant Professor of the Biochemistry and Biophysics department and a Principal Investigator of the Cardiovascular Research Institute at the University of California San Francisco. In 2012, he was named Scholar of the Pew Charitable Trust. Dr. D’Angelo was recruited to Sanford Burnham Prebys in October 2014.
Funding Awards and Collaborative Grants
Pew Charitable Trust Scholar in Biomedical Sciences
Related Disease
Aging-Related Diseases
Nuclear pore complexes (NPCs) are multiprotein channels that penetrate the nuclear envelope and act as the gatekeepers of the genome. NPCs work together with nuclear transport receptors to ferry molecules in and out of the nucleus. They also regulate multiple cellular processes such as gene expression, chromatin organization, and RNA processing in a transport independent manner. In recent years, our lab and others identified that cells can change the configuration of nuclear pore complexes to modulate specific cellular processes, such as T cell homeostasis and activation, muscle differentiation, tumor development and metastasis.
Because of the essential function of nuclear pore complexes in cell survival, proliferation and differentiation, it is not surprising that changes in the nuclear transport machinery have long been observed in cancer cells. Yet, how most of these alterations contribute to cell transformation and tumor development is poorly understood.
Our laboratory is working to:
- Establish how alterations in the nuclear transport machinery contribute to cancer development and progression
- Dissect the role of nuclear pore complexes in the regulation of immune cell function
- Identify, validate, and modulate new therapeutic targets for cancer and immune disorders
Our long-term goal is to develop novel therapies targeting the cellular nuclear transport machinery.
 Mar 31, 2025
Mar 31, 2025Delicate balancing act determines how many genome gateways form in cells
Mar 31, 2025Study finds that protein creation and disposal systems control the amount of nuclear pore complexes in cells.
 Jun 5, 2024
Jun 5, 2024How a protein component of nuclear pore complexes regulates development of blood cells and may contribute to myeloid disorders
Jun 5, 2024Nuclear pore complexes (NPCs) are channels composed of multiple proteins that ferry molecules in and out of the nucleus, regulating…
 Sep 28, 2020
Sep 28, 2020Scientists kill cancer cells by “shutting the door” to the nucleus
Sep 28, 2020Proof-of-concept study shows that stopping the construction of nuclear pore complexes selectively kills cancer cells. Scientists at Sanford Burnham Prebys…
 Jun 5, 2017
Jun 5, 2017Muscle-enhancing protein could hold key to treating muscular dystrophies
Jun 5, 2017Nuclear pore complexes are large multiprotein channels that act as the sole gateway between the nucleus and cytoplasm. For many…
Nicholas Cosford, PhD has served on the Sanford Burnham Prebys Board of Trustees since 2023. He is the first faculty member to do so.
Cosford joined the Sanford Burnham Prebys faculty in 2008 as an associate professor. In 2013, he became a full professor. His lab investigates the interactions of small molecule compounds with therapeutically important proteins and cellular signaling pathways. With a specific focus on the discovery and optimization of compounds that might treat cancer, central nervous system diseases and infectious diseases.
Prior to joining Sanford Burnham Prebys in 2005, Cosford worked in both the biotechnology and pharmaceutical industries. At Sibia Neurosciences and at Merck Research Laboratories, he directed multidisciplinary research teams focused on small-molecule hit-to-lead optimization and was responsible for moving several lead compounds through to the clinical phase, including a nicotinic agonist (Altinicline) from research to Phase II clinical trials for treating Parkinson’s disease.
He is an author of more than 90 peer-reviewed, published scientific papers, and has been issued more than 40 issued patents, with an additional 40 patent applications pending.
Cosford has a Bachelor of Science degree in chemistry from the University of Bath in England and Doctor of Philosophy degree in organic chemistry from Emory University in Atlanta, GA.
Related Disease
Alzheimer’s Disease, Amyotrophic Lateral Sclerosis (Lou Gehrig’s Disease), Bone Mineralization Disorders, Breast Cancer, Cancer, Neurodegenerative and Neuromuscular Diseases, Neurological and Psychiatric Disorders, Ovarian Cancer, Pancreatic Cancer, Prostate Cancer
We are interested in investigating the interactions of small molecule compounds with therapeutically important proteins and cellular signaling pathways. One aspect of our research emphasizes the use of medicinal chemistry and chemical biology approaches to probe intracellular pathways that regulate cell survival and cell growth. Another area of active research is the development of synthetic chemistry methodology using microfluidic technology for the rapid synthesis of biologically active small molecules. Therapeutically, we are primarily focused on the discovery and optimization of compounds that have the potential to treat cancer, CNS diseases and infectious diseases.
 Aug 2, 2024
Aug 2, 2024Sanford Burnham Prebys event explores the science behind addiction
Aug 2, 2024Scientists and clinicians from three local research institutions converged July 31 to discuss new ways to treat multiple addictions at…
 Jul 25, 2024
Jul 25, 2024The Science Behind Addiction
Jul 25, 2024Scientists and clinicians from three local research institutions converge July 31 to discuss new ways to treat multiple addictions at…
 Nov 8, 2023
Nov 8, 2023From tobacco to alcohol to opioids, Sanford Burnham Prebys researchers are pursuing novel leads and promising therapies to treat addiction
Nov 8, 2023Addiction is perhaps the most and least visible of public health crises in the US. Tens of millions of Americans…
 Mar 20, 2023
Mar 20, 2023Padres Pedal the Cause 2023: Team Sanford Burnham Prebys raises $50,000 for cancer research
Mar 20, 2023Team Sanford Burnham Prebys hit the pavement this weekend for Padres Pedal the Cause, an annual fundraising event that invites…
 Mar 20, 2023
Mar 20, 2023Sanford Burnham Prebys researchers awarded Curebound grants
Mar 20, 2023Each year, Sanford Burnham Prebys joins Padres Pedal the Cause, an annual fundraising event that raises money for Curebound which…
 Mar 9, 2023
Mar 9, 2023Sanford Burnham Prebys elects Professor Nicholas Cosford to its Board of Trustees
Mar 9, 2023Sanford Burnham Prebys today announced that Professor Nicholas Cosford, PhD, will join the Institute’s Board of Trustees. “Scientists are the
Dr. Colas earned his PhD from the Universite Pierre et Marie Curie, Paris, France.
Funding Awards and Collaborative Grants
2011-2013: American Heart Association Post-Doctoral Fellowship
2011: Best Talk Award (Development & Aging Post-Doctoral Retreat, Sanford Burnham Prebys)
2010-2011: California Institute for Regenerative Medicine Post-Doctoral Fellowship
2006-2007: French Myopathy Association PhD Fellowship
2003-2006: Ministry of French Research PhD Fellowship
2000-2001: Erasmus Undergraduate Fellowship
Related Disease
Cardiomyopathies, Cardiovascular Diseases, Heart Disease
Phenomena or Processes
Cardiovascular Biology, Cell Biology, Development and Differentiation, Embryogenesis, Embryonic/Pluripotent Stem Cells, Regenerative Biology, RNA-Based Gene Regulation, Transcription Factors
Our laboratory focuses on the identification and reconstruction of novel regulatory pathways controlling the determination and the acquisition of cardiovascular cell fates in humans. To that end, our team has developed a unique set of cell-based assays suitable for high-throughput functional screening, which enables the implementation of unbiased and systematic experimental approaches with unprecedented exploratory power. Our goal is to leverage generated knowledge to produce better cardiomyocytes to model cardiac diseases in-a-dish as well as to devise innovative cardiac regenerative strategies. In parallel, our lab is also dedicated to identify and develop new drugs promoting cardiac regeneration and/or helping preserving heart function after injury.
 Oct 8, 2024
Oct 8, 2024Alexandre Colas named 2024 Mentor of the Year at Sanford Burnham Prebys
Oct 8, 2024Colas was applauded for enthusiastically promoting mentees’ professional and scientific development.
 Aug 15, 2023
Aug 15, 2023Scientists unite to get to the heart of AFib
Aug 15, 2023A collaborative study led by Institute researchers is paving the way to identifying gene networks that cause atrial fibrillation (AFib),…
 Mar 29, 2023
Mar 29, 2023Heart attack study could change the game in regenerative medicine
Mar 29, 2023Institute researchers have identified a group of proteins that could be the secret to cellular reprogramming, an emerging approach in…
 Jun 21, 2021
Jun 21, 2021Alexandre Colas awarded $1.9 million for atrial fibrillation research
Jun 21, 2021Scientist will use novel platform to identify drugs that restore normal cardiac rhythm Sanford Burnham Prebys Assistant Professor Alexandre Colas has been
 Aug 23, 2019
Aug 23, 2019NIH grant aims to boost heart muscle
Aug 23, 2019Heart disease is the number one killer of Americans. Now, the National Institutes of Health (NIH) has awarded a four-year…
Lukas Chavez is an Associate Professor at the Sanford Burnham Prebys. He is also the Director of the Clayes Research Center for Neuro-Oncology at the Institute for Genomic Medicine at the Rady Children’s Hospital, San Diego. In this role, he works with a team of physicians and scientists to capture genomic, transcriptomic, epigenetic and functional data from pediatric brain tumor patients, and uses this information to improve diagnosis and treatment. His research interests focus on structural variants as well as circular extrachromosomal DNA (ecDNA) in childhood cancers. These extrachromosomal DNA circles are frequently found in highly aggressive solid tumors and represent a new target for improved therapeutic approaches.
Education
2010: PhD, Free University, Berlin
Honors and Recognition
2020: St. Baldrick’s Scholar Award, St. Baldrick’s Foundation
2019: Award of Excellence in Pediatric Neuro-Oncology, Society of Neuro-Oncology
2012–2015: Feodor-Lynen Fellowship for Postdoctoral Researchers, Alexander-von-Humboldt Foundation
Related Disease
Brain Cancer, Cancer
Phenomena or Processes
Cancer Biology, Cancer Epigenetics, Chromosome Dynamics, Combinatorial Therapies, Gene Regulation, Genomic Instability, Oncogenes, Transcriptional Regulation
Anatomical Systems and Sites
Brain
Research Models
Clinical and Transitional Research, Computational Modeling, Cultured Cell Lines, Human, Human Cell Lines, Mouse
Techniques and Technologies
Bioinformatics, Cell Biology, Computational Biology, Computational Modeling, Gene Expression, Gene Silencing, Genomics, Single Nucleotide Polymorphisms (SNPs)
 Aug 15, 2025
Aug 15, 2025Zombie cancer cells give cold shoulder to chemotherapy
Aug 15, 2025Study shows that combining chemotherapy with targeted treatment for these lumbering cells improves outcomes in mice.
 Aug 13, 2025
Aug 13, 2025Chavez joins NIH Cancer Genetics Study Section
Aug 13, 2025Lukas Chavez, PhD, has been named a standing member of the Cancer Genetics Study Section at the National Institutes…
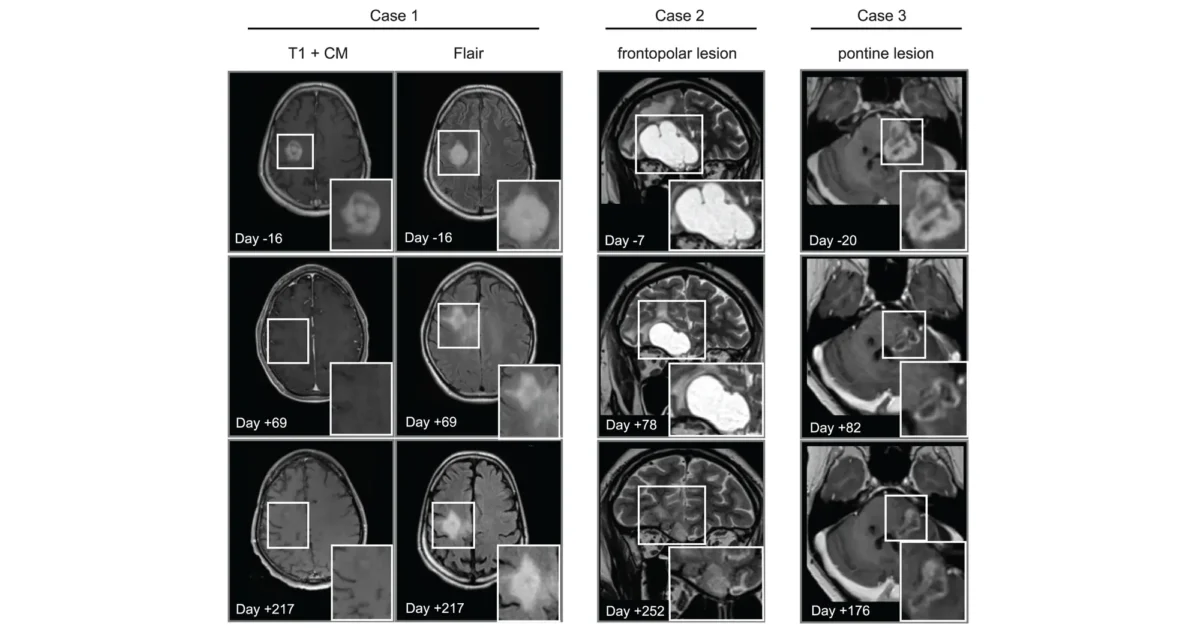 Apr 14, 2025
Apr 14, 2025Cancer drug finds new purpose in the brain
Apr 14, 2025Scientists show that a cancer drug travels to and shrinks some brain tumors, which may lead to new therapies.
 Aug 6, 2024
Aug 6, 2024Coding clinic
Aug 6, 2024Rapidly evolving computational tools may unlock vast archives of untapped clinical information—and help solve complex challenges confronting healthcare providers
 Jul 30, 2024
Jul 30, 2024Using machines to personalize patient care
Jul 30, 2024Artificial intelligence (AI) and other computational techniques are aiding scientists and physicians in their quest to create treatments for individuals…
 Jul 17, 2024
Jul 17, 2024Sanford Burnham Prebys announces new faculty recruit and two faculty promotions
Jul 17, 2024Douglas Sheffler was named as a new associate professor at Sanford Burnham Prebys, while Cosimo Commisso and Nicholas Cosford garnered…
Dr. Bradley received her doctorate from the University of California Berkeley, in 1981 in studies of CD4 T cell subsets that regulate humoral immune responses. Her work on the regulation of CD4 T cells continued during her postdoctoral training at The Oregon Primate Research Center and at the University of California, San Diego where she was appointed Assistant Research Professor in 1991. It was at this time she developed NIH sponsored her research program on CD4 T cells and discovered the key associations between migration and function. She joined The Scripps Research institute as an Assistant Professor in 1996 where she expanded her work on CD4 T cells into the arena of autoimmunity and discovered the essential role of the cytokine, interleukin-7, in the regulation of CD4 cell homeostasis.
She joined the Sidney Kimmel Cancer Center in 2001 as an Associate Professor, and was promoted to Professor in 2005. She joined Sanford Burnham Prebys as a Professor in the Infectious and Inflammatory Diseases Center in 2009. Dr. Bradley is recognized as a key contributor in the field of CD4 T cell biology, is an invited speaker at many national and international meetings, and serves on several study sections for the NIH as well as the Welcome Trust, Medical Research Council, and the JDRF.
Related Disease
Cancer, Infectious Diseases, Skin Cancer and Melanoma
Phenomena or Processes
Adaptive Immunity, Cell Signaling, Infectious Disease Processes, Inflammation
Anatomical Systems and Sites
Immune System and Inflammation
The research program in the Bradley lab is focused on understanding the regulation of T lymphocytes in virus infections where the immune response results in viral clearance and the development of immunologic memory, and in chronic virus infections where the ongoing immune response leads to viral persistence and immune dysregulation. They are guided by these studies to interrogate cellular mechanisms that can be modulated to promote better responses not only to virus infections, but also to relieve immune inhibition in the setting of cancer where T cells progressively lose function. Understanding adhesion mechanisms underlie the ability of T cells to become localized in tissues to eradicate infections and tumors is a key underpinning of their work.
The Bradley lab’s current focus is on two molecules that can function on T cells to initiate the processes that lead to their migration from the blood into tissue, CD44 and PSGL-1 (P-selectin glycoprotein-1). They have found that both of these receptors have key regulatory functions that are independent of their roles in migration. These proteins regulate the magnitude of T cell responses, as well as the survival and memory formation by T cells by different mechanisms, affecting processes in the T cell and stromal cell compartments.
Their ongoing studies of these immune checkpoint regulators using in vivo models indicate that they are promising therapeutic targets to enhance T cell responses to infections and cancer as well as to inhibit T cell responses in autoimmunity. They are therefore pursuing translational studies with the UCSD Moores Cancer center to analyze their regulation in human T cells in an effort to enhance patient responses to their tumors using in vivo modeling. In addition working to develop biologics for treatment of patients with autoimmunity and cancer.
 Aug 19, 2024
Aug 19, 2024Women in Science event at Sanford Burnham Prebys examines how female faculty members navigate research careers
Aug 19, 2024Topics at the event included work/life balance, caregiving and family obligations, and gender disparities in academic rank at research and…
 Mar 26, 2024
Mar 26, 2024Melanoma’s mysteries revealed at Sanford Burnham Prebys
Mar 26, 2024Cancer Center open house welcomes San Diego community to learn the latest about melanoma research.
 May 3, 2023
May 3, 2023Reviving exhausted T cells to tackle immunotherapy-resistant cancers
May 3, 2023A new approach to immunotherapy could help overcome treatment resistance in cancer. When the cells of our immune system are…
 Mar 20, 2023
Mar 20, 2023Sanford Burnham Prebys researchers awarded Curebound grants
Mar 20, 2023Each year, Sanford Burnham Prebys joins Padres Pedal the Cause, an annual fundraising event that raises money for Curebound which…
- Mar 19, 2018
Cancer immunology symposium highlights hot area in cancer research
Mar 19, 2018The Cancer Immunology and Tumor Microenvironment Symposium held at Sanford Burnham Prebys Medical Discovery Institute (SPB) on March 8, 2018…
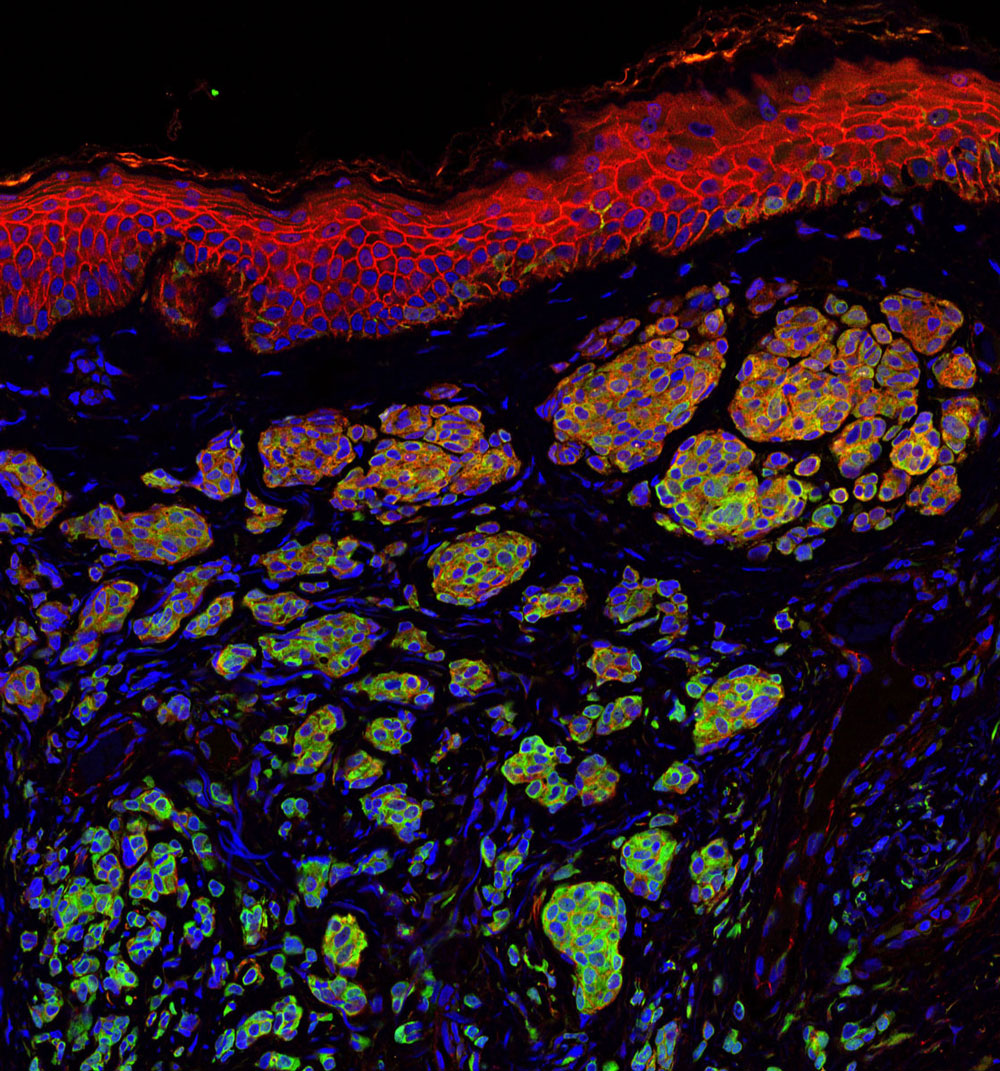 May 16, 2017
May 16, 2017What SBP Scientists are Researching to Battle Skin Cancer
May 16, 2017Skin cancer is one of the most common of all cancers, and melanoma accounts for about 1 percent of skin…
Rolf Bodmer earned his PhD in Biochemistry and Neurobiology from the University of Basel, Switzerland, in 1983. Dr. Bodmer trained as a postdoctoral fellow in Neurobiology at the Albert Einstein College of Medicine in New York, and also studied Molecular Genetics at the University of California, San Francisco. He was appointed Assistant Professor of Biology in 1990 at the University of Michigan. There, he was promoted to Associate Professor of Biology in 1996, and then appointed to Associate Professor of Molecular, Cellular and Developmental Biology in 2001. Dr. Bodmer joined Sanford Burnham Prebys in 2003, where he is Professor and Director of the Center for Cardiovascular and Muscular Diseases.
Other Appointments
Adjunct professor, University of California, San Diego
Funding Awards and Collaborative Grants
1 P01 AG033561 “Genetic Analysis of Drosophila Functional Aging”
Honors and Recognition
Ellison Foundation Senior Scholar Award
Related Disease
Cancer, Cardiovascular Diseases, Heart Disease, Inherited Disorders, Metabolic Diseases, Metabolic Syndrome, Muscular Dystrophy, Neurodegenerative and Neuromuscular Diseases, Obesity, Parkinson’s Disease
The Bodmer lab is interested in the molecular mechanisms of organ formation, how patterns are generated and how cells and tissue types assume their correct fates and functions. The Bodmer lab is pursuing this interest by studying the genetic functions and interactions that specify heart development and maintain heart performance in the Drosophila model, in the hope of elucidating basic principles in organogenesis and functionality.
Mesoderm
↓
Cardiac Mesoderm
↓
Cardiac Cell Types
↓
Morphogenesis
↓
Heart Function
↓
Aging Heart
They study the HIF and Notch pathways in various organismal responses to hypoxia. Both of these pathways as well as mechanisms and responses to hypoxia are of high relevance to cancer research.
They are also studying master regulatory networks in how they control metabolism and obesity. These fundamental studies on obesity pathways will also be highly relevant to cancer metabolism.
 Jul 17, 2023
Jul 17, 2023New genes implicated in deadly heart defect
Jul 17, 2023By identifying genes in patients and testing their effects in fruit flies, Institute researchers have found new genes that contribute…
 Dec 14, 2020
Dec 14, 2020Our top 10 discoveries of 2020
Dec 14, 2020This year required dedication, patience and perseverance as we all adjusted to a new normal—and we’re proud that our scientists
 Jun 22, 2020
Jun 22, 2020Scientists uncover new genetic mutations linked to autism spectrum disorder
Jun 22, 2020The study opens new research avenues for the condition. Scientists at Sanford Burnham Prebys Medical Discovery Institute and Radboud University Medical Center
 Dec 4, 2019
Dec 4, 2019A year in review: Our top 10 discoveries of 2019
Dec 4, 2019At Sanford Burnham Prebys, we uncover the origins of disease and launch bold new strategies that lay the foundation for…
 May 23, 2019
May 23, 2019Epigenetic change causes fruit fly babies to inherit diet-induced heart disease
May 23, 2019Scientists are learning that epigenetic changes, or molecular tags that modify our DNA, can cause your children to inherit the…
 Feb 15, 2019
Feb 15, 20195 takeaways from Insights: Heart Disease
Feb 15, 2019It’s easy to forget about the fist-sized organ in our chest. But the heart is arguably the most important muscle