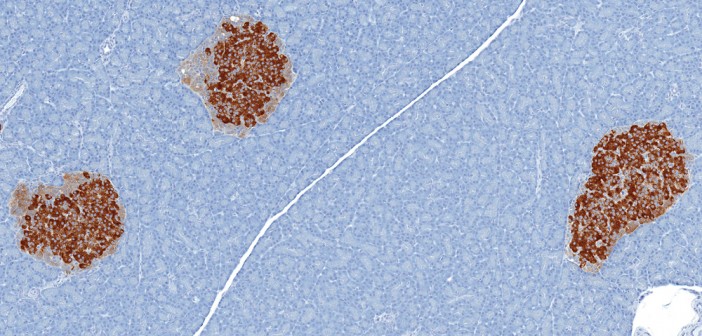
Type 2 diabetes is a chronic disease that affects about eight percent of adults worldwide, significantly increasing the risk of heart disease and stroke. This disease interferes with the body’s ability to make or use a hormone called insulin, which is produced by beta cells in the pancreas. These cells eventually fail in many patients with type 2 diabetes, making insulin replacement therapy a necessity for survival. However, this treatment is imprecise, onerous and often promotes weight gain, highlighting the strong need for better treatment options.
Toward that goal, Sanford-Burnham researchers recently shed new light on the precise mechanisms leading to beta cell failure. In a recent study published in Diabetes, they showed that defective protein folding in beta cells leads to oxidative stress—a process that triggers programmed cell death. Treatment with antioxidants or a molecule that promotes proper protein folding preserved beta cells in mice. The findings open promising new avenues for the treatment of type 2 diabetes in humans.
“For a long time, it has been suggested that oxidative stress is a principal contributing factor for beta cell death and dysfunction. However, the detailed mechanism was unclear until now,” said senior study author Randal Kaufman, PhD, director of the Degenerative Diseases Program at Sanford-Burnham. “The findings have implications for all diseases of protein misfolding, from Alzheimer’s disease, to inflammatory bowel disease, to diabetes, to fatty liver disease and to cancer.”
Reducing stress improves symptoms Insulin helps to move a blood sugar called glucose into cells to be stored for energy. High blood glucose levels resulting from abnormal insulin signaling can damage nerves and blood vessels, potentially leading to complications such as heart disease and stroke, which are leading causes of death worldwide. Beta cells try to compensate for deficient insulin responses by producing more insulin, putting extra stress on an organelle called the endoplasmic reticulum to properly fold, process, and secrete the hormone.
Past research by the Kaufman lab has shown that an increase in protein synthesis in beta cells causes the accumulation of misfolded proteins in the endoplasmic reticulum, in addition to oxidative stress—a process that can lead to cell damage and death through the build-up of toxic molecules called reactive oxygen species. But until now, the mechanisms by which protein misfolding in the endoplasmic reticulum initiates a cell death response were not known.
In the new study, Kaufman and his team found that mice with a mutation that causes protein misfolding in the endoplasmic reticulum develop diabetes due to decreased beta cell function and mass, oxidative stress and cell death. Treatment with a chemical chaperone—a molecule that promotes proper protein folding—improved beta cell function and reduced diabetes symptoms in these mice, suggesting that endoplasmic reticulum stress is responsible for beta cell failure. Moreover, treatment with antioxidants reduced oxidative damage and thereby restored beta cell function, increased insulin secretion and improved glucose metabolism. Taken together, the findings suggest that protein misfolding in the endoplasmic reticulum causes oxidative stress, leading to beta cell failure in type 2 diabetes.
“Based on our previous studies, we expected that antioxidants would prevent beta cell failure,” Kaufman said. “What was surprising is how quickly this occurred—within one week. As far as I know, no interventions can so rapidly reverse beta cell function and restore glucose homeostasis in diabetic mice.”
Moving forward, Kaufman and his team will investigate exactly how antioxidant treatment rapidly reverses diabetes in mice and identify additional antioxidants that are expected to be safe and effective in humans. “Our findings suggest that antioxidant or chemical chaperone treatment may be a promising therapeutic approach not only for metabolic disease, but also neurodegenerative diseases, inflammatory diseases, and cancer,” Kaufman said. “By further examining the underlying molecular mechanisms, we are optimistic that our research will lead to exciting new treatments for these common, debilitating diseases.”
To read the paper in full use this link. Janelle Weaver, PhD, a freelance writer, contributed to this article.