Jamey Marth is a Professor at Sanford Burnham Prebys. Dr. Marth’s previous positions included Professor of Medical Genetics at the Biomedical Research Centre, University of British Columbia; Investigator of the Howard Hughes Medical Institute and Professor of Cellular and Molecular Medicine at the University of California San Diego; and Professor and Director of the Center for Nanomedicine at the University of California Santa Barbara. Dr. Marth received a PhD degree in Pharmacology from the University of Washington where he trained in the laboratories of Roger M. Perlmutter, MD, PhD, and Nobel-laureate Edwin G. Krebs, MD.
Education
1987: PhD, University of Washington, Pharmacology
1984: BSc, University of Oregon, Genetics and Chemistry
Honors and Recognition
2024: Distinguished Service Award, Society for Glycobiology
2017: Karl Meyer Award, Society for Glycobiology
2009-2020: John Carbon Chair in Biochemistry and Molecular Biology
2009-2019: Duncan and Suzanne Mellichamp Chair in Systems Biology
2009: Julius Stone Lectureship Award: Society for Investigative Dermatology
1995-2009: Investigator Award, Howard Hughes Medical Institute
1991-1995: Faculty Scholarship, The Medical Research Council of Canada
Related Disease
Cancer, Colitis, Diabetes – General, Inflammatory/Autoimmune Disease, Sepsis
Dr. Marth is a molecular and cellular biologist specializing in diseases attributable to protein glycosylation. His education and training span molecular genetics, biochemistry, pharmacology, cell biology, immunology, hematology, developmental biology, microbiology, and glycobiology.
As an enzymatic process essential to cells, glycosylation produces saccharides linked by glycosidic bonds to proteins, lipids, and themselves, termed glycans. The vast majority of secreted and cell surface proteins are post-translationally modified by glycosylation during transit through the secretory pathway, termed glycoproteins. A widely used college level cell biology textbook authored by others denotes glycans as one of the four main families of the organic molecules of all cells, with lipids, proteins, and nucleic acids. Together they compose the macromolecules and other assemblies of the cell. The structures of glycans (and lipids) are, however, synthesized by template-independent processes, rendering them hard to predict and study. However, cells produce and regulate an abundant and diverse glycome of glycosidic linkages in which some of the biological information is decoded by one or more glycan-binding receptors, termed lectins.
Glycans and lectins represent a significant percentage of genes in the genomes of organisms, with several hundred present in mammals. Because glycan biosynthesis, diversification, and degradation rely upon corresponding gene and enzyme function, glycan function can be investigated similarly to other enzymatic and metabolic pathways, such as protein phosphorylation. In contrast however, studies of intact organisms are typically required to uncover the functions of protein glycosylation in mammals. Dr. Marth’s laboratory identified this model system requirement and has further focused on discovering how glycosidic linkages regulate proteins modified by N- and O-glycans and contribute to the molecular origins of common diseases and syndromes including colitis, diabetes, autoimmune disease, and sepsis.
To understand the nature and extent of the information within glycosidic linkages, the Marth Laboratory has applied multiple molecular approaches to investigate protein glycosylation in mice and humans. This includes the development of enabling technologies with broad applicability, such as conditional mutagenesis by Cre-lox recombination in living animals to determine gene function with temporal and spatial selectivity. His laboratory also develops and studies disease models that better represent real-world models of environmental factors that trigger common acquired human diseases. The laboratory is based on interdisciplinary research focused upon physiology and disease process regulated by protein glycosylation.
The physiological systems regulated by protein glycosylation are broad even when comparing among sequential biosynthetic steps, and our findings continue to indicate the presence of undiscovered information of medical relevance residing in the glycan linkages of glycoproteins.
 Jul 31, 2025
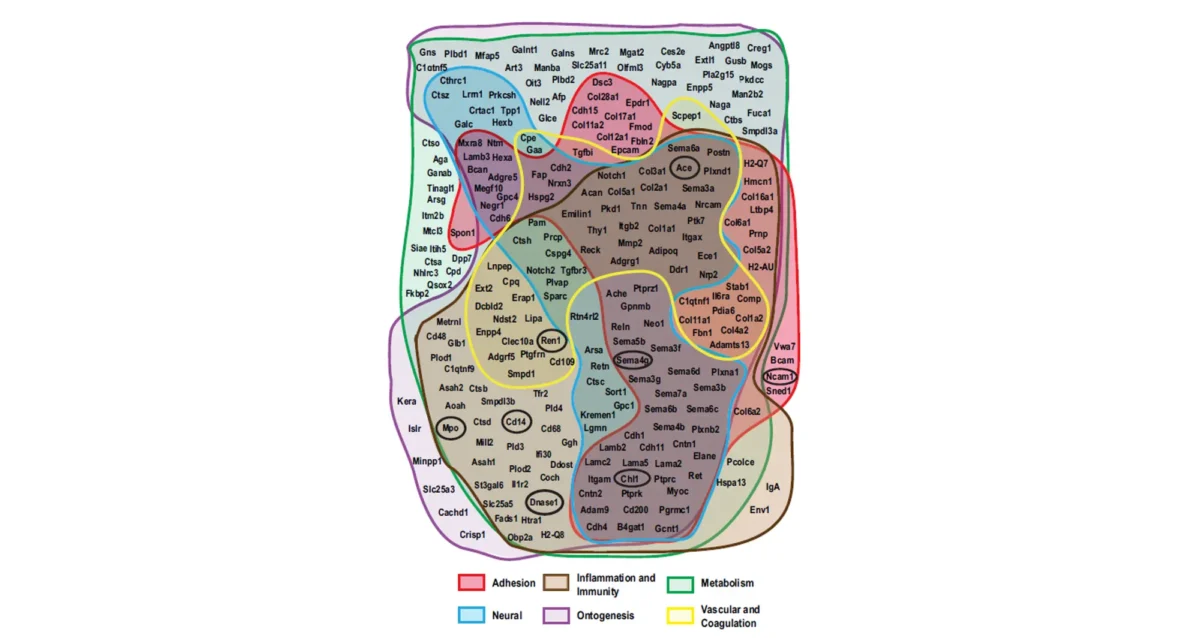
Jul 31, 2025Signal boost uncovers hundreds of hidden binding partners for blood protein receptor
Jul 31, 2025Study identifies receptor-ligand interactions, links receptor dysfunction to age-associated defects in multiple organs.
 May 14, 2025
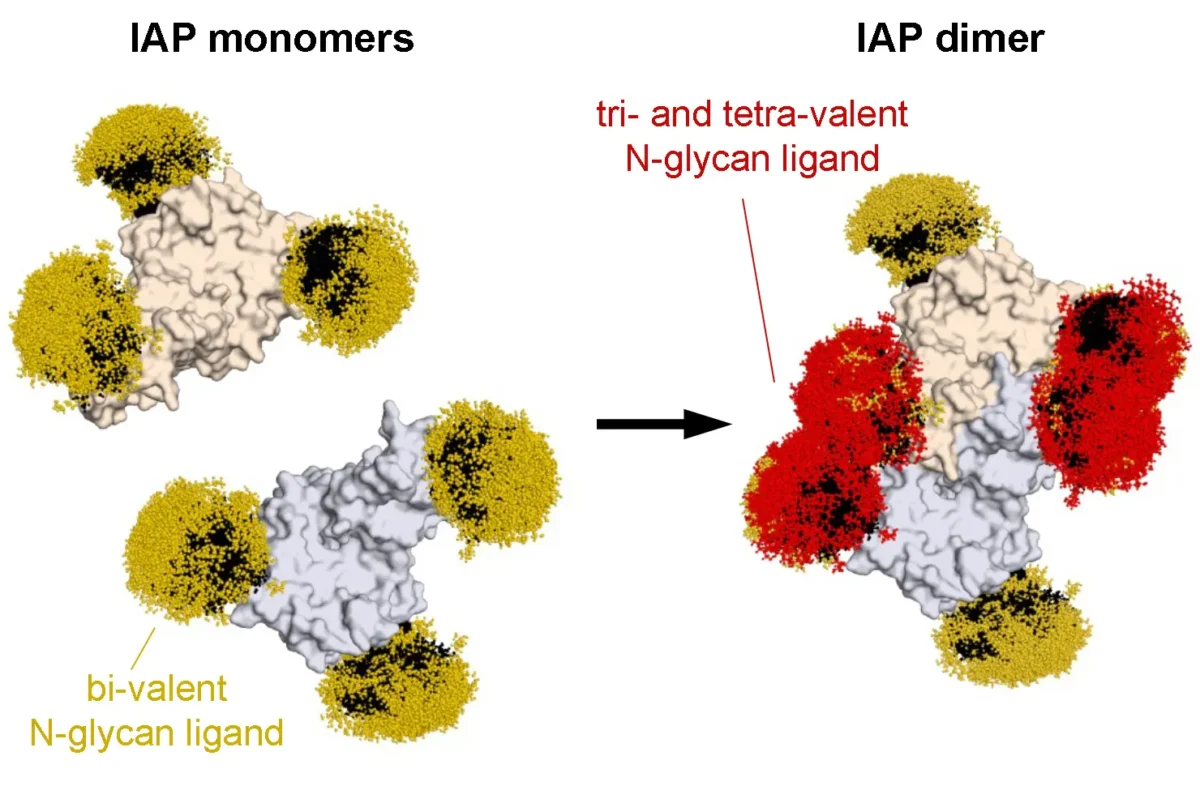
May 14, 2025Rediscovering the first known cellular receptor
May 14, 2025Scientists from the Marth lab apply new techniques to reexamine a receptor linked to sepsis.
 Oct 31, 2024
Oct 31, 2024Jamey Marth interviewed by The Scientist
Oct 31, 2024The Sanford Burnham Prebys scientist discussed the Cre-loxP recombination system, a mainstay genetic engineering technology.
 Sep 28, 2021
Sep 28, 2021Jamey Marth awarded $13.5 million by NIH to investigate the pathogenesis and treatment of sepsis
Sep 28, 2021Sanford Burnham Prebys professor Jamey Marth, PhD, has been awarded $13.5 million from the National Heart, Lung, and Blood Institute to
 Jul 13, 2021
Jul 13, 2021Study finds promising therapeutic target for colitis
Jul 13, 2021Neu3 controlled the emergence of disease in a model of human colitis An international research group, led by Jamey Marth, PhD,
 Oct 10, 2017
Oct 10, 2017Jamey Marth honored for research linking glycans to diabetes, lupus, sepsis
Oct 10, 2017Jamey Marth, Ph.D., is the 2017 recipient of the Society for Glycobiology’s Karl Meyer Award. The international award is given…
