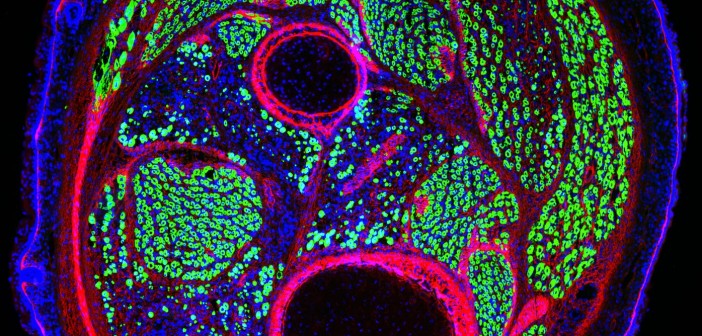
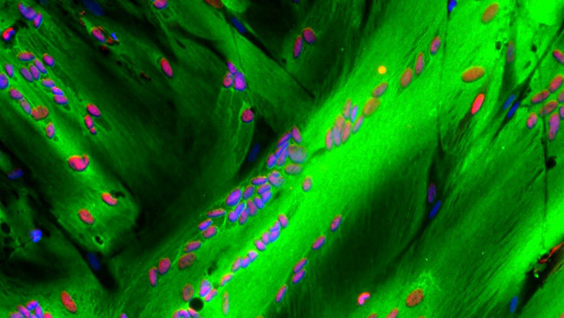
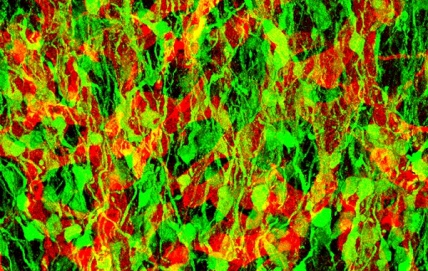
Researchers in Alessandra Sacco’s lab have found a key to enhancing repair of damaged muscle. In work published in Cell Reports, they describe why fetal muscle stem cells (MuSCs) are better at regenerating muscle compared to adult MuSCs. The research opens the door for new approaches to treat muscle diseases including muscular dystrophies, which affect approximately 50,000 people in the U.S., and muscle wasting associated with cancer and aging.
“We found that fetal MuSCs remodel their microenvironment by secreting specific proteins, and then examined whether that same microenvironment can encourage adult MuSCs to more efficiently generate new muscle. It does, which means that how adult MuSCs normally support muscle growth is not an intrinsic characteristic, but can be changed,” explained Matthew Tierney, a graduate student at SBP and first author of the study.
The proteins that fetal MuSCs secrete are part of the extracellular matrix (ECM), the meshwork of strand-like proteins and starches that make up the structure of MuSCs’ microenvironment. As fetal MuSCs mature into adult MuSCs, they take on different responsibilities and help change their microenvironment over time to support their distinct functions. Fetal MuSCs are geared toward creating new muscle, whereas adult MuSCs repair damaged muscle and self-replicate to sustain the pool of stem cells to mend future injuries.
In muscular dystrophies and muscle wasting, progressive degeneration overwhelms the regenerative capacity of adult MuSCs. The new study showing that adult MuSCs living in a microenvironment with fetal characteristics are better at regenerating muscle provides rationale for developing drugs that could trigger this transition.
“These results help explain the differences between the capacity of fetal and adult MuSCs to repair muscle. Such an understanding is urgently needed, as no treatments are yet available for muscular dystrophies and muscle-wasting disorders,” stated Alessandra Sacco, PhD, associate professor in the Development, Aging, and Regeneration Program at SBP and senior author of the study.
“Our findings fit with the growing appreciation of the importance of a cell’s structural and biochemical surroundings in influencing cellular behavior. Managing the microenvironment is an emerging approach to treat many diseases, from cancer to cardiovascular disease to neurodegeneration. We’re excited about the implications of our research for treating muscle diseases, and look forward to applying our conclusions toward development of therapies.”
The full text of the paper is available here.