New Sanford Burnham Prebys scientist investigates the mutational powers of cancer cells — and their vulnerabilities
Growing up in China, the world’s second most populous country at 1.425 billion people, Xueqin “Sherine” Sun, PhD, says she was acutely aware of the presence of cancer and its consequences. Almost one-quarter of new cancer cases each year occur in China, and one in three deaths.
“I have seen the enormous disability, suffering and mortality caused by cancer. As it becomes more prevalent globally, I believe that almost everyone has or will experience cancer at personal level, in their families or among friends. I’ve lost many relatives and friends to cancer and I have always been curious about what is cancer, why people get cancer and why we cannot treat cancer like other curable diseases?”
As a new assistant professor at Sanford Burnham Prebys faculty, Sun seeks to better understand the genetic and epigenetic underpinnings of cancers, using genome editing technologies, animal and patient-derived models, and other tools to develop more effective cancer therapies.
She’s already helped make progress. Working previously with Alea Mills, PhD, a professor at the National Cancer Institute-designated cancer center at Cold Spring Harbor Laboratory in New York, Sun co-published findings last year explaining why a gene called P53, generally regarded as protective against malignancies, sometimes suffers its own debilitating that renders it powerless against the deadliest type of brain tumors called glioblastomas.
“My lab is interested in studying how DNA or the machinery that interprets it leads to the transformation of normal cells into cancerous cells and concurrently, their specific vulnerabilities. Identifying these intrinsic vulnerabilities and targeting them properly is profoundly important to developing effective cancer therapies.”
Another aspect of Sun’s work is understanding how cancer cells and tumors change their circumstances and environment to improve survival, including hiding from or repressing the immune system.
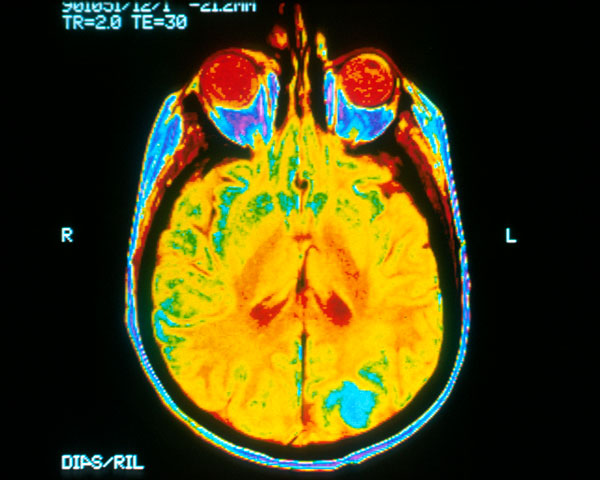
A colorized magnetic resonance image of a patient with brain cancer depicts a glioblastoma or tumor (blue) metastasizing in the occipital lobe (bottom of MRI). Image courtesy of National Cancer Institute.
“Changes to DNA itself and the way how DNA is interpreted by cells can transform normal cells into cancer cells,” says Sun. “And transformed cells propagate by enhancing the misinterpreted DNA information, which in turn becomes the Achilles’ heel of cancer cells. Our goal is to find out how DNA information is misinterpreted in different ways and how to correct it to halt cancer.”
At Sanford Burnham Prebys, Sun and colleagues will employ a host of leading-edge tools and approaches, including functional genomics, artificial intelligence, structural biology, large-scale drug screening, and advanced imaging/spatial technologies.
“I’m super excited to leverage these powerful tools in our basic cancer research, drug development and in vivo assessment of therapeutic interventions. I think the biggest challenge in cancer treatment is the heterogeneity that contributes to drug resistance and tumor relapse.
“By leveraging these techniques, we can gain more insights into cancer at the single cell resolution, develop novel drugs faster, deliver drugs to cancer cells more efficiently and selectively, predict the potential evolutionary trajectory of cancer upon treatment and eliminate persistent tumor cells before recurrence.
“These endeavors will help shift the paradigm for clinical practice in diagnosis, decision-making, medication administration, outcome evaluation and level up precision medicine.”
