Dr. Ani Deshpande’s most recent position was as an Instructor with Dr. Scott Armstrong at the Memorial Sloan Kettering Cancer Center and the Children’s Hospital Boston/Harvard Medical School. He completed his PhD in Human Biology from the Ludwig Maximillians University in Munich. Dr. Deshpande’s research revolves around studying difficult-to-cure leukemias through the use of mouse models, genomic and epigenomic studies.
Funding Awards and Collaborative Grants
Ongoing Research Support R00 phase (number in process) Deshpande (PI) 10/0/14-present NIH/NCI – K99/R00 Howard Temin Pathway to Independence Award Role: PI (75% effort) Completed Research Support K99 CA154880 Deshpande (PI) 07/15/11-09/30/14 NIH/NCI – K99/R00 Howard Temin Pathway to Independence Award Role: Post-doctoral fellow/PI
Honors and Recognition
2014: American Society of Hematology Scholar Award (ASH Junior Faculty Scholar Award)
2013: Alex’s Lemonade Stand Foundation Travel Award for the FASEB Hematological Malignancies Meeting in Vermont, VA
2013: Abstract Achievement Award, American Society of Hematology Annual Meeting, New Orleans
2012: Abstract Achievement Award, American Society of Hematology Annual Meeting, Atlanta
2008: ASH Travel Award: 50th Annual Meeting, American Society of Hematology (ASH) San Diego
2008: The New York Stem Cell Foundation (NYSCF) Travel Grant, International Society of Stem Cell Research (ISSCR), Philadelphia
2007: The George Brecher New Investigator Award (postdoctoral) of the International Society of Experimental Hematology, Hamburg, Germany
2007: Doctoral prize of the German Society of Hematology and Oncology (Annual prize for the best doctoral thesis in hematology-oncology in Germany)
2007: The Doctoral Prize of the Helmholtz Centre, Munich for the Best Doctoral Thesis 2006, Munich, Germany
2007: Best Poster Award (2nd Prize): 36th Annual Scientific Meeting, International Society for Experimental Hematology (ISEH) Hamburg
2007: Travel Award: 36th Annual Scientific Meeting, International Society for Experimental Hematology (ISEH) Hamburg
2006: Summa Cum Laude Ludwigs Maximililans University, Munich, Germany
2005: ISEH Travel Grant: 34th Annual Scientific Meeting, International Society for Experimental Hematology (ISEH) Glasgow
2004: ISEH Travel Grant: 33rd Annual Scientific Meeting, International Society for Experimental Hematology (ISEH) New Orleans
2003: ASH Travel Award: 45th Annual Meeting, American Society of Hematology (ASH) San Diego
Related Disease
Cancer, Leukemia/Lymphoma
One of the core interests of the lab is to understand regulation of key developmental processes that are important for normal stem cells and are frequently misregulated in human cancer. We are currently investigating genetic and epigenetic abnormalities in Acute Myeloid Leukemia (AML) using mouse models, gene targeting and genome-scale approaches. Our overarching goal is to uncover therapeutic nodes of intervention in human leukemia.
Available Positions in the Deshpande Lab
We are seeking exceptional candidates with broad interests/expertise in cancer epigenetics and stem cell biology at the level of postdoctoral fellows, graduate students and undergraduates.
- Graduate students
Applicants interested in graduate positions should send their applications to the Sanford Burnham Prebys Medical Discovery Institute Graduate School for Biomedical Sciences. The application period opens in the fall. - Undergraduate students
Please contact Dr. Deshpande via email adeshpande@sbpdiscovery.org.
Ani Deshpande’s Research Report
Normal and malignant stem cells
One of the core interests of the lab is to investigate the role of chromatin regulators in benign and malignant hematopoiesis. Several attributes of normal stem cells such as the ability to self-renew are co-opted or reactivated by cancer cells on their way to malignant transformation. We are interested in characterizing the molecular determinants of “stemness” using hematopoietic stem cells as a model and in identifying ways and means by which these stem-cell associated pathways are usurped for oncogenesis.
The leukemia epigenome
Abnormal epigenetic changes have emerged as important mediators of oncogenesis. Genomic investigations of human cancer have uncovered mutations in writers, erasers and readers of the histone code. The goal of our laboratory is to connect basic mechanisms of chromatin regulation to diseased states with a focus on Acute Myeloid Leukemia (AML). Ultimately, we are interested in the rational design of screens to develop therapeutics targeting dysregulated epigenetic mechanisms in AML.
Chromosomal translocations in cancer
Ever since the discovery of the Philadelphia chromosome in 1960, a number of different chromosomal translocations have been identified in human cancer that result in the formation of potent fusion oncogenes, abnormal activation of latent proto-oncogenes, or other oncogenic events. We are very interested in investigating the impact of chromosomal translocations on the development of human cancer. Moreover, we are also interested in understanding the propensity of certain genomic loci for recurrent involvement in oncogenic chromosomal translocation events in the context of human myeloid malignancies.
 Feb 9, 2026
Feb 9, 2026How scientists turned plant poisons into medicines
Feb 9, 2026Podcast details how caustic compounds meant to deter eaters of plants were harnessed to treat diabetes.
 Nov 14, 2025
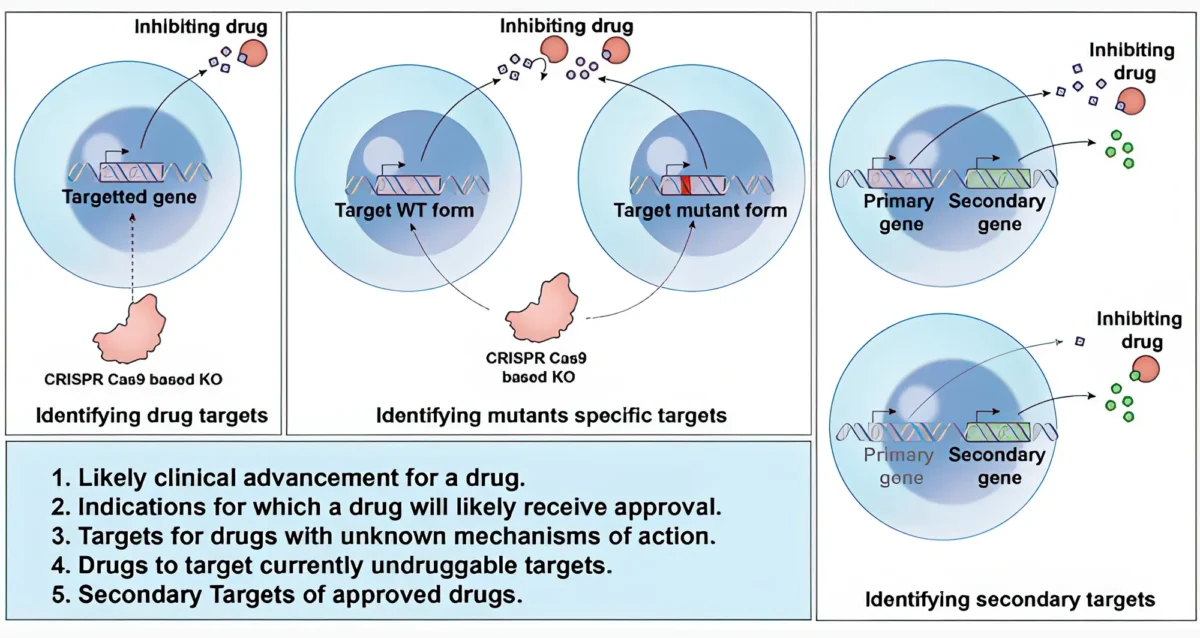
Nov 14, 2025Computational deep dive surfaces unexplored world of cancer drug targets
Nov 14, 2025DeepTarget tool predicts direct and indirect targets of cancer drugs.
 Sep 24, 2025
Sep 24, 2025How a Holocaust survivor transformed blood sugar testing
Sep 24, 2025New podcast discusses how a scientist saved from a World War II concentration camp revolutionized diabetes care.
 Sep 8, 2025
Sep 8, 2025Sizing up a weakness in synovial sarcoma’s genes
Sep 8, 2025Study shows that studying cancer’s genetic vulnerabilities can lead to new potential targeted therapies for patients.
 Aug 19, 2025
Aug 19, 2025Seeing how sugar reshapes our blood
Aug 19, 2025Scientists and podcasters tackle how a scientist in Iran overcame great odds and forever changed diabetes diagnoses.
 Jul 16, 2025
Jul 16, 2025Ani Deshpande promoted to professor in the Cancer Genome and Epigenetics Program at Sanford Burnham Prebys
Jul 16, 2025Deshpande will continue studying how blood cancers sabotage stem cells’ special features to grow and spread.
