Dr. Commisso obtained his PhD at the University of Toronto in the Department of Molecular Genetics and completed his postdoctoral training at New York University School of Medicine. Dr. Commisso made the seminal discovery that Ras-mutant cancer cells use macropinocytosis as an amino acid supply pathway. His laboratory’s research interests are centered on elucidating the underlying mechanisms of how metabolic stress influences the tumor ecosystem in pancreatic cancer. The lab is particularly interested in identifying metabolic adaptations and dependencies that contribute to tumor progression and can be targeted to develop novel therapeutic modalities for this disease.
Related Disease
Cancer, Colorectal Cancer, Lung Cancer, Pancreatic Cancer
Research in the Commisso lab is focused on biological discoveries that have the potential to lead to novel therapeutic strategies for cancer. Of particular interest to our laboratory are Ras-driven cancers, such as pancreatic cancer, which are extremely aggressive and are in urgent need of new and innovative therapies. The biological process that we study in the lab is called macropinocytosis, a fluid-phase form of bulk endocytic uptake, which we have linked to cancer cell metabolism in Ras-mutated tumors.
Cosimo Commisso’s Research Report
Macropinocytosis is an endocytic mechanism of fluid-phase uptake that produces large intracellular vesicles known as macropinosomes. Macropinosomes are heterogeneous in size and shape and serve to internalize large volumes of extracellular fluid along with the associated membrane. In transformed cells, macropinocytosis is stimulated by oncogenes, such as Ras. Ras proteins are small, membrane-localized GTPases that are activated in response to growth factors and they regulate a variety of outputs, including cell proliferation, survival and invasion. Gain-of-function mutations in Ras-encoding genes cause Ras proteins to be trapped in their active state, leading to the constitutive activation of downstream pathways. The functional consequences of macropinocytosis stimulation in mutant Ras-expressing cells were unknown prior to our work. We have linked macropinocytic uptake in Ras-transformed cells to nutrient delivery and amino acid supply (Commisso et al., 2013). We demonstrated that the inhibition of this nutrient delivery pathway selectively compromises growth of Ras-driven tumors. With the long-term goal of specifically targeting such tumors, we have recently developed stream-lined methodology to detect and grade macropinocytosis in tumor tissue (Commisso et al., 2014). Our work was important for two main reasons. First, cancer cells are dependent on amino acids, such as glutamine, for their growth and survival. Therefore, the targeting of these amino acid supply pathways, such as macropinocytosis, represents a promising strategy in developing anti-cancer therapeutics. Second, macropinocytosis is emerging as a mechanism of entry for a variety of therapeutic agents, such as nanoparticles. Hence, identifying that this uptake pathway is active in Ras-driven tumors may have an impact on how these tumors are treated. The complete understanding of the functional significance of Ras-induced macropinocytosis to carcinogenesis and treatment ultimately depends on having a firm grasp of how this process is regulated and on the ability to specifically control it. To this end, a major research focus of the lab is to advance our understanding of the molecular pathways that drive macropinocytosis, which could lead to the identification of new molecular targets whose inhibition would restrain tumor growth and enhancers that could be manipulated to dial-up the uptake process in drug delivery strategies. Additional research interests in the lab include nutrient sensing pathways that are active in the context of macropinocytic uptake and macropinosome maturation, the process that leads to active protein catabolism within the tumor cell.
 Nov 10, 2025
Nov 10, 2025Factoring in frailty and age to improve pancreatic cancer treatment
Nov 10, 2025Study is first to show how aging allows pancreatic tumors to grow and spread faster, may lead to new personalized…
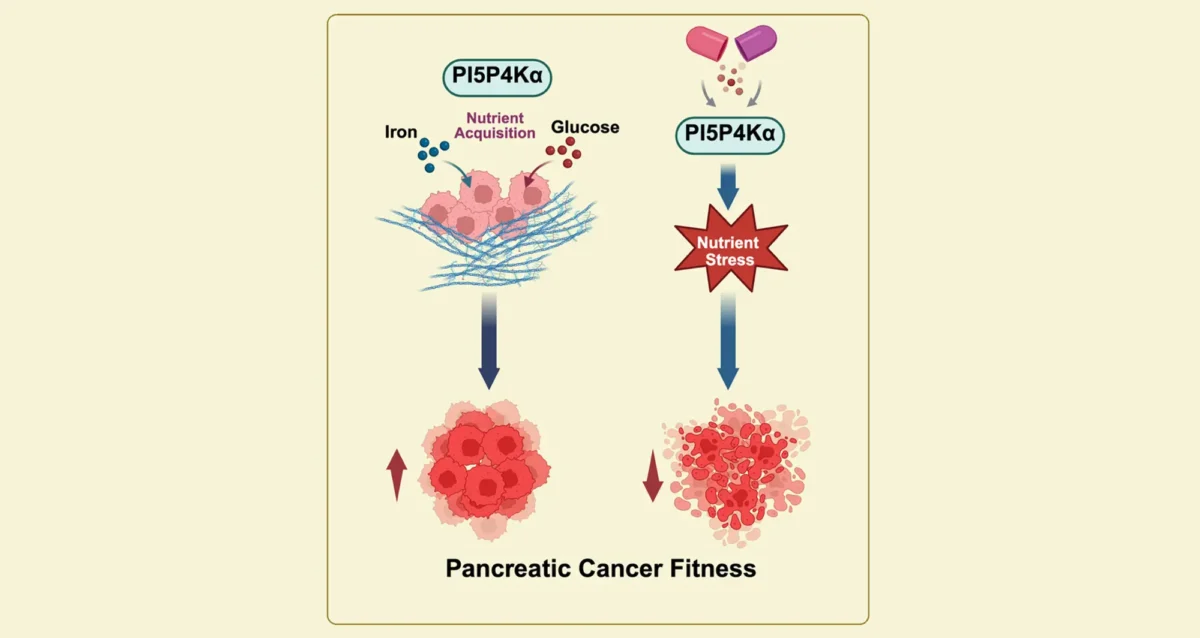 Sep 11, 2025
Sep 11, 2025Starving Cancer Cells of Essential Sugar and Iron
Sep 11, 2025Findings from Gurpreet Kaur Arora, Brooke Emerling, and Cosimo Commisso, identify key enzyme that pancreatic cancer cells need to obtain…
 Jul 24, 2025
Jul 24, 2025Reshaping tumor neighborhoods to give treatments a boost
Jul 24, 2025Blocking a fuel source changes tissue near pancreatic tumors, enabling more effective immunotherapy and chemotherapy.
 Dec 5, 2024
Dec 5, 2024Controlling cancer cells’ gluttony for glutamine
Dec 5, 2024Scientists discover new insights about how pancreatic cancer cells adapt when nutrients and other resources are scarce.
 Oct 18, 2024
Oct 18, 2024Two Sanford Burnham Prebys scientists selected for American Cancer Society postdoctoral fellowships
Oct 18, 2024The funds will support Alicia Llorente Lope and Ambroise Manceau who study breast and pancreatic cancer.
 Jul 17, 2024
Jul 17, 2024Sanford Burnham Prebys announces new faculty recruit and two faculty promotions
Jul 17, 2024Douglas Sheffler was named as a new associate professor at Sanford Burnham Prebys, while Cosimo Commisso and Nicholas Cosford garnered…

