Dr. Yu Xin (Will) Wang received his PhD at the University of Ottawa where he identified cellular asymmetry and polarity mechanisms regulating muscle stem cell self-renewal and skeletal muscle regeneration. He then carried out postdoctoral training at Stanford University School of Medicine developing single cell multi-omic approaches to characterize the regenerative process and what goes awry with disease and aging.
“I’ve always had a passion for science and became fascinated with how the body repairs and heals itself when I was introduced to the potential of stem cells in regenerative medicine. I was struck by the ability of a small pool of muscle stem cells that can rebuild and restore the function of muscle. My lab at Sanford Burnham Prebys aims to better understanding the repair process and harness our body’s ability to heal in order to combat chronic diseases and even counteract aging.“
Education and Training
Postdoctoral Fellowship, Stanford University School of Medicine
PhD in Cellular Molecular Medicine, University of Ottawa, Canada
BS in Biomedical Sciences, University of Ottawa, Canada
Prestigious Funding Awards
2020: NINDS K99/R00 Pathway to Independence Award
Honors and Recognition
Governor General’s Gold Medal – Canada
Related Disease
Aging-Related Diseases, Amyotrophic Lateral Sclerosis (Lou Gehrig’s Disease), Arthritis, Cachexia, Inflammatory/Autoimmune Disease, Multiple Sclerosis, Muscular Dystrophy, Myopathy, Neurodegenerative and Neuromuscular Diseases, Sarcopenia/Aging-Related Muscle Atrophy, Spinal Muscular Atrophy
Phenomena or Processes
Adult/Multipotent Stem Cells, Aging, Cell Signaling, Development and Differentiation, Epigenetics, Exercise, Extracellular Matrix, Neurogenesis, Organogenesis, Regenerative Biology, Transcriptional Regulation
Anatomical Systems and Sites
Immune System and Inflammation, Musculoskeletal System, Nervous System
Research Models
Clinical and Transitional Research, Computational Modeling, Human Adult/Somatic Stem Cells, Mouse
Techniques and Technologies
3D Image Analysis, Bioinformatics, Cellular and Molecular Imaging, Gene Knockout (Complete and Conditional), Genomics, High Content Imaging, High-Throughput/Robotic Screening, Live Cell Imaging, Machine Learning, Microscopy and Imaging, Proteomics, Transplantation
The Wang lab is interested in elucidating critical cell-cell interactions that mediate the function of tissue-specific stem cells during regeneration and disease, with a focus on how a coordinated immune response can promote regeneration and how autoimmunity impacts tissue function and hinder repair.
Specifically, the Wang lab aims to identify cellular and molecular crosstalk between muscle, nerve, and immune systems to develop targeted therapies that overcome autoimmune neuromuscular disorders and autoimmune aspects of “inflammaging.”
Yu Xin (Will) Wang’s Research Report
The lab’s research is translationally oriented and utilizes interdisciplinary molecular, genetic, computational (machine learning and neural networks), and bioengineering approaches to view biology and disease from new perspectives. We combine multi-omics sequencing and imaging methods to resolve how different cell types work together after injury to repair tissues and restore function. We use a data-driven approach to identify targetable disease mechanisms and, through collaborations with other researchers and clinicians, develop therapies that promote regeneration. Visit our lab website to learn more.
 Jun 12, 2025
Jun 12, 2025Turning back time on muscle stem cells to prevent frailty from aging
Jun 12, 2025Study from Dr. Will Wang’s lab finds a way to restore stem cells from aged muscle to become young again…
 Aug 20, 2024
Aug 20, 2024Mapping the human body to better treat disease
Aug 20, 2024Scientists are investigating the inner workings of our bodies and the cells within them at an unprecedented level of detail.
 Aug 13, 2024
Aug 13, 2024Dodging AI and other computational biology dangers
Aug 13, 2024Sanford Burnham Prebys scientists say that understanding the potential pitfalls of using artificial intelligence and computational biology techniques in biomedical…
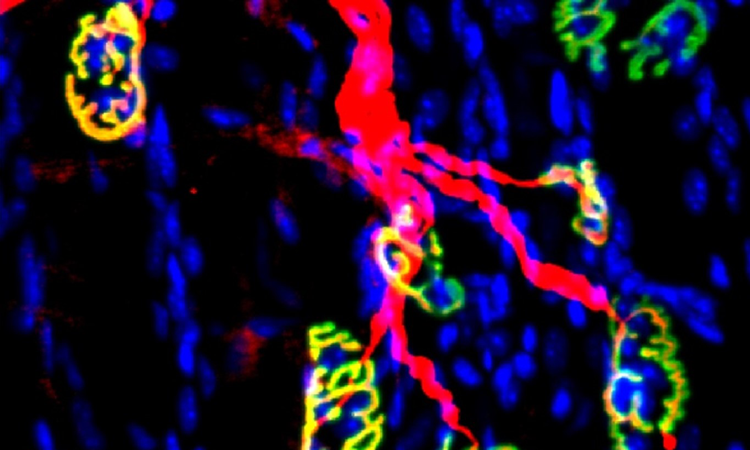 Oct 11, 2023
Oct 11, 2023Inhibiting an enzyme associated with aging could help damaged nerves regrow and restore strength
Oct 11, 2023New research has demonstrated a way to accelerate recovery from peripheral nerve injury by targeting an enzyme that was thought…
 Jan 26, 2023
Jan 26, 2023Three big questions for cutting-edge biologist Will Wang
Jan 26, 2023Will Wang’s spatial omics approach to studying neuromuscular diseases is unique.
 Nov 23, 2022
Nov 23, 2022Yu Xin (Will) Wang joins Sanford Burnham Prebys to advance regenerative medicine
Nov 23, 2022Molecular biologist Yu Xin (Will) Wang, PhD, has joined Sanford Burnham Prebys as an assistant professor in the Development, Aging,…
