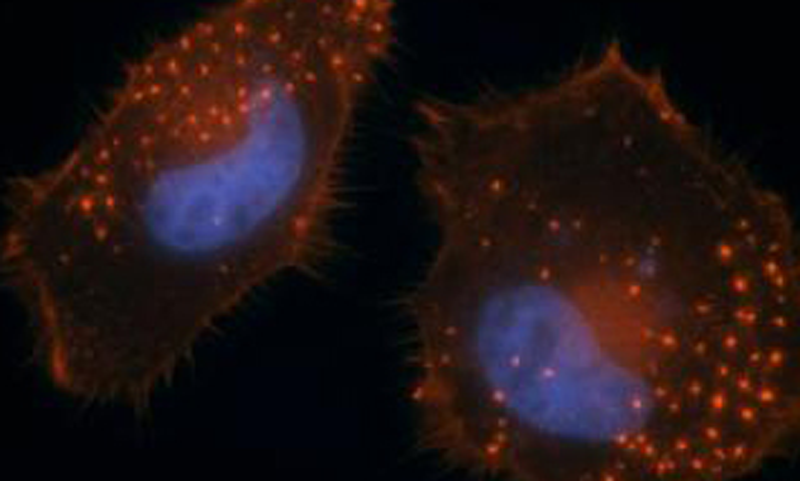
Metastasis--the spread of cancer from the place where it first started to another place in the body--is the most common reason that cancer treatments fail. To metastasize, some types of cancer cells rely on invadopodia, cellular membrane projections that act like feet, helping them "walk" away from the primary tumor and invade surrounding tissues. To determine how cells control invadopodia formation, scientists at Sanford-Burnham Medical Research Institute (Sanford-Burnham) screened a collection of pharmacologically active compounds to identify those that either promote or inhibit the process. The study, led by Sara Courtneidge, PhD and postdoctoral researcher Manuela Quintavalle, PhD in collaboration with scientists in Sanford-Burnham's Conrad Prebys Center for Chemical Genomics (Prebys Center), revealed compounds that inhibit invadopodia formation without causing toxicity. The search also turned up several other compounds that increased the number of invadopodia.
Two major findings came out of this research. First, several of the newly identified invadopodia inhibitors targeted a family of enzymes called cyclin-dependent kinases (Cdks), revealing a previously unrecognized role for Cdks in invadopodia formation. Secondly, one of the pro-invadopodia compounds was the chemotherapeutic agent paclitaxel--a finding that might have implications for the drug's current use in treating cancer. These findings will appear online July 26 in Science Signaling.
"Previous studies by our group and others have demonstrated that we might be able to target invadopodia to prevent cancer cell invasiveness," said Dr. Courtneidge, professor and director of the Tumor Microenvironment Program in Sanford-Burnham's NCI-Designated Cancer Center. "In this study, we established a cell-based screening assay to help us identify regulators of invadopodia formation."
Dr. Courtneidge's group has been studying invadopodia for a number of years with the goal of unraveling how they regulate tumor cell invasion. Sanford-Burnham's Prebys Center provided them with expertise in chemical genomics, the robotic technology necessary to rapidly and reproducibly screen more than 1,000 compounds with known pharmacological activity in cell-based assays, and automated microscopy capable of detecting and measuring invadopodia formation.
This screening study identified several compounds that block invadopodia, and therefore cancer cell invasion. The team was surprised to find that many of these compounds targeted Cdks, a family of enzymes that were not previously associated with invadopodia. In follow-up experiments, the researchers demonstrated that one of these enzymes, Cdk5, is required for the formation and function of invadopodia and for cellular invasion, important steps in cancer metastasis. Cdk5 is highly expressed in neurons, where it's involved in neuronal migration and outgrowth, but this is the first time the enzyme has been implicated in invadopodia formation.
Taking the study a step further, Drs. Courtneidge and Quintavalle and the team also worked out how Cdk5 promotes invadopodia formation. Cdk5's action leads to the degradation of another protein called caldesmon. Caldesmon was previously shown to negatively regulate invadopodia, so Cdk5 essentially removes that brake. That's why the Cdk inhibitors identified in the screening study also inhibited invadopodia.
Another pharmacologically active compound shown by the screen to regulate invadopodia was paclitaxel, a drug currently used to treat patients with many forms of cancer. Paclitaxel's anti-tumor activity is based on its ability to bind and stabilize microtubules, one component of the cellular cytoskeleton, thereby halting cell division and inducing cellular suicide. In this study, paclitaxel promoted invadopodia formation and cancer cell invasion. This makes sense because invadopodia formation also depends on microtubules, which are stabilized by paclitaxel. These results raise the concern that continued treatment with paclitaxel might be counterproductive in cancer patients who aren't responding well to the drug or in cases where the tumor has not yet been removed. Moreover, paclitaxel could actually provoke cancer metastasis in these patients.
"Although our results suggest paclitaxel might increase metastasis, we also observed that the drug did not promote invasive behavior in cells treated with an invadopodia inhibitor," said Dr. Courtneidge. "This defines a potential clinical path for testing inhibitors in the context of paclitaxel treatment. In other words, a patient could still benefit from paclitaxel's cancer cell-killing effect if physicians also have the ability to add a therapeutic invadopodia inhibitor when resistance develops."
This study provides the proof-of-concept that the identification of invadopodia regulators might also lead to new strategies for controlling metastatic cancer growth.