Alexander Strongin earned his PhD from Moscow State University in Russia in 1972 and his D.Sci. degree from the Institute of Microbial Genetics in Moscow in 1983. From 1982 to 1988, Dr. Strongin was head of the Laboratory of Functional Enzymology at the Institute of Genetics of Microorganisms in Moscow. He served as head of the Department of Biotechnology and Laboratory of Protein Engineering, Institute of Molecular Genetics, Russian Academy of Sciences, Moscow, from 1988 to 1990. From 1990 to 1994, he was a visiting professor of biochemistry in the Division of Dermatology at Washington University School of Medicine, St. Louis, Missouri. Dr. Strongin has worked in the La Jolla area since 1994, as senior staff scientist in the Biology Division at General Atomics, 1994-1995, and as senior staff scientist at the La Jolla Institute for Experimental Medicine, 1995-1999. Dr. Strongin joined Sanford Burnham Prebys on September 1, 1999.
Related Disease: Type 1 Diabetes
Dr. Levine is Emeritus Professor at Sanford Burnham Prebys. Prior to that, he was a Professor in the Department of Pediatrics at the University of California, San Diego School of Medicine, where he continues to see children with inherited metabolic diseases. Dr. Levine received his undergraduate degree in biochemistry from Harvard and his MD and PhD degree in genetics from the University of Washington. His clinical training as a pediatric geneticist was at the Children’s Hospital of Philadelphia. Dr. Levine has been working in the field of cell transplantation therapies for diabetes and b-cell biology for more than fifteen years. His laboratory was the first to develop immortalized cell lines from the human endocrine pancreas as models of beta-cell growth and differentiation. He has made insights into cellular senescence in the endocrine pancreas, finding that b-cells undergo rapid senescence when stimulated to proliferate. Most recently, he and his co-workers demonstrated the existence of endocrine stem cells in the adult human pancreas. The laboratory continues to pursue the development of cell therapies for diabetes using a variety of approaches, including high throughput screening.
Education
1979-86: PhD, University of Washington (Genetics)
1979-86: MD, University of Washington
1975-79: A.B., Harvard University (Biochemistry)
Postgraduate Training
1989-91: Genetics Fellow, Dept. of Pediatrics, UCSD School of Medicine
1988-89: Clinical Genetics Fellow, Children’s Hosp. of Philadelphia
1987-89: Pediatric Resident, Children’s Hosp. of Philadelphia
1986-87: Pediatric Intern, Children’s Hosp. of Philadelphia
Other Appointments
Health Sciences Clinical Professor of Pediatrics, UCSD School of Medicine
Attending Physician, Rady Children’s Hospital
Related Disease
Cancer, Childhood Diseases, Metabolic Diseases, Obesity, Type 1 Diabetes, Type 2 Diabetes
Diabetes is a disease in which there is an insufficient amount of insulin, produced by the pancreatic b-cells, to control the blood glucose concentration. In type I diabetes, the b-cell are destroyed by an autoimmune response, while in type II diabetes, the b-cells are dysfunctional and are ultimately lost because of obesity-associated pathology. Our laboratory is interested in how b-cells regenerate under normal and pathophysiological conditions, with the goal of developing new therapies for diabetes that result in an increased number of those cells. Our studies of b-cell growth have also led us to develop lead compounds that are active in cancer, an interesting connection as both obesity and diabetes are now recognized as major risk factors for cancer.
Fred Levine’s Research Report
The major interest in the laboratory is pancreatic beta-cell biology and specifically the control of beta-cell growth and differentiation in the adult pancreas. In both type I and type II diabetes, β-cell mass decreases and is a major factor in the pathogenesis of diabetes. Thus, one major question in which we are interested is the existence and nature of adult endocrine stem/progenitor cells. Using both human and rodent models, we study cells in vitro and in vivo to determine the competence of various cell populations to undergo endocrine differentiation in response to defined stimuli. The goal is to achieve a sufficient understanding of the process of adult β-cell regeneration to allow us to enhance β-cell mass in diabetes.
A second major area of emphasis is to use high-throughput screening to discover compounds that modulate important aspects of β-cell biology. Our focus has been on modulation of insulin promoter activity, as that gene is acted upon by many diabetogenic stimuli. We have isolated and are characterizing a number of compounds and genes that were discovered in the screening process. Recently, we have discovered ligands for the orphan nuclear receptor HNF4a. HNF4a is involved in a number of disease processes, including diabetes, liver disease, and cancer.
A career history of fundamental discovery and translational research in immunology has guided Dr. Ware to identify new drug targets and develop novel therapeutics. Dr. Ware’s career in immunology and virology began in 1982 when he became a Professor at the University of California, Riverside’s Division of Biomedical Sciences. In 1996, he joined the La Jolla Institute for Immunology in San Diego as Head of the Division of Molecular Immunology. Professor Ware joined Sanford Burnham Prebys Medical Discovery Institute in 2010, serving as the Director of the Infectious and Inflammatory Disease Center and Adjunct Professor of Biology at the University of California at San Diego. He is currently the Director of the Laboratory of Molecular Immunology, which focuses on discovering and designing immunotherapeutics.
As an educator, he taught medical students immunology and virology. He trained over 60 postdoctoral fellows and graduate students who chose careers in research in academic and pharmaceutical science, patent law, or teaching.
Dr. Ware advises scientific panels and review boards for the National Institutes of Health and serves on the scientific advisor boards for the Allen Institute for Immunology and the Arthritis National Research Foundation. Scientific advisor with several biotechnology and pharmaceutical companies on immunotherapy for cancer and autoimmune diseases using innovative approaches to target discovery and drug development.
Dr. Ware’s research program is dedicated to unraveling the intricate intercellular communication pathways that govern immune responses. His work, which centers on cytokines in the Tumor Necrosis Factor (TNF) Superfamily, particularly those that regulate cell survival and death in response to viral pathogens, spans the domains of cancer,autoimmune and infectious diseases.
At Sanford Burnham Prebys, Dr. Ware is pivotal in promoting the translation of the faculty’s scientific discoveries. His efforts have led to the Institute’s reputation as a productive and preferred partner in collaborations with Pharma, including multi-year research and drug development projects with Eli Lilly and Avalo Therapeutics. His success translating fundamental knowledge into rational drug design has led to three novel therapeutics targeting inflammatory pathways, currently in clinical trials.
Education
1981-1982: T cell Immunology, Dana-Farber Cancer Institute of Harvard Medical School in Boston, MA. Tim Springer and Jack Strominger, advisors.
1979-1981: Biochemistry of Complement, University of Texas Health Science Center, San Antonio, TX. W Kolb, advisor
1974-1979: PhD in Molecular Biology and Biochemistry from the University of California, Irvine; Gale Granger, PhD mentor.
Honors and Recognition
Distinguished Fellow, American Association of Immunologists
Honorary Lifetime Membership Award International Cytokine and Interferon Society
Hans J. Muller-Eberhardt Memorial Lecture
Biotech All Star, San Diego Padres Awar
“Pillars of Immunology” discovery of the Lymphotoxin-b Receptor, published in Science
Outstanding Alumnus, Ayala School of Biological Sciences, University of California, Irvine
National MERIT Award R37 (10 years), National Institute of Allergy and Infectious Disease, NIH
National Research Service Award, NIH Postdoctoral Fellowship
Related Disease
Arthritis, Breast Cancer, Cancer, Crohn’s Disease (Colitis), Infectious Diseases, Inflammatory Bowel Disease, Inflammatory/Autoimmune Disease, Inherited Disorders, Leukemia/Lymphoma, Myeloma, Pathogen Invasion, Psoriasis, Systemic Lupus Erythematosus, Type 1 Diabetes
Research in the Laboratory of Molecular Immunology is directed at defining the intercellular communication pathways controlling immune responses. Our work is focused on the Tumor Necrosis Factor (TNF)-related cytokines in regulating decisions of cell survival and death, especially in responses to viral pathogens. Translational research is redirecting the communication networks of TNF superfamily to alter the course of autoimmune and infectious disease and cancer.
Carl Ware’s Research Report
Discovery of the TNF-LIGHT-LTαβ Network
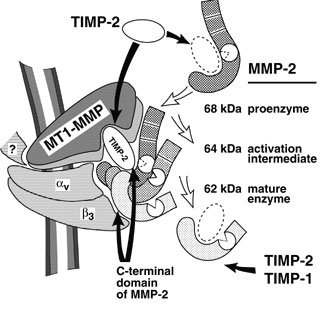
The molecular elements of this cellular communication network were revealed with our discovery of Lymphotoxin-β and its formation of the trimeric heterocomplex with LTαβ1 and its signaling receptor, Lymphotoxin-β Receptor2. The LTβR revealed a new inter-cellular communication pathway that provides a key mechanism underlying the development and homeostasis of lymphoid organs. A second ligand we discovered, LIGHT (TNFSF14), is a novel ligand for the herpesvirus entry mediator (HVEM; TNFRSF14), and surprisingly, the LTβR3 heralding the concept that TNF, LTαβ, and LIGHT form an integrated signaling network thru distinct receptors controlling inflammation and host defense.4
LTβR Signaling in Host Defense and Immune Evasion
Our investigations into the mechanisms of virus evasion of the immune system revealed an essential role of the LTβR pathway in regulating the type 1 interferon response to cytomegalovirus5 and now recognized as a major defense against other pathogens. LTβR signals differentiate macrophages and stromal cells into IFN-producing cells. LTβR transcriptomics and proteomics datasets we generated revealed a novel constellation of anti-viral host defense mechanisms6. Importantly the role of the LTβR pathway to alter tissue microenvironments by differentiation of specialized stromal cells has implications for promoting effective immune responses to cancer.
Discovery of the HVEM-BTLA Immune Checkpoint
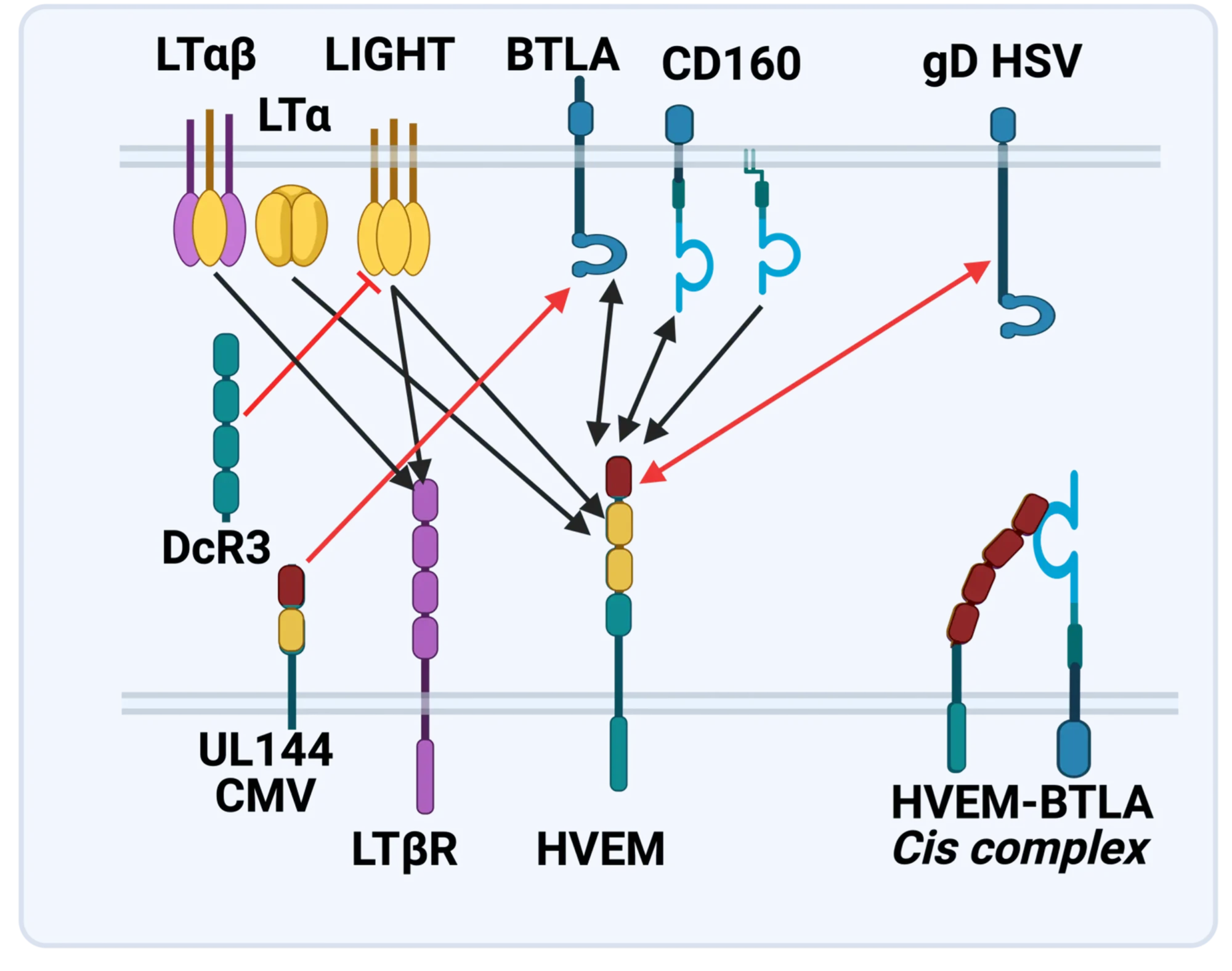
The LTBR-HVEM-BTLA Network in the TNF Superfamilies. Arrows indicate ligand-receptor binding (black), inhibitors (red arrow) and bidirectional signaling (dual arrowheads); HSV, herpes simplex virus; CMV, human cytomegalovirus. BTLA and CD160 are Ig Superfamily proteins. BTLA is an inhibitory checkpoint receptor; DcR3, decoy receptor 3 inhibits LIGHT binding to HVEM and LTBR.
The discovery that HVEM is a coreceptor for the immune checkpoint, B and T lymphocyte attenuator (BTLA), an Ig superfamily member, established a new paradigm in TNF Receptor signaling pathways 7. Additional investigations revealed the importance of the HVEM-BTLA system in limiting immune responses, including T cell help for B cell clonal expansion, antibody maturation, and secretion8. HVEM-BTLA also regulates control of the intestinal microbiome, limiting invasion of pathogenic bacteria and enhancing Treg cell homeostasis 9. The diverse roles of this pathway are seen in the loss of BTLA signaling from mutations in HVEM frequently present in B cell lymphomas10. Additional layers of immune regulators, CD160 and DcR3, control the LIGHT-HVEM-BTLA pathways, revealing this network as a key mechanism controlling immune homeostasis.
Appreciating the fundamental features of the TNF-LIGHT-LTab Network in effector and homeostasis mechanisms presents a target-rich resource for therapeutic intervention in autoimmunity, infection, and cancer11, 12.
Translational research and Immunotherapy
- 2021-current: Lead Scientific Advisor, Avalo Therapeutics
A neutralizing, fully human mAb (quisovalimab) to the proinflammatory cytokine LIGHT (TNFSF14) completed Phase I with an excellent safety profile and a Phase II trial establishing efficacy in COVID-19 pneumonia (NCT0441205)13. We identified elevated serum levels of LIGHT in hospitalized patients with COVID1914 spurring a randomized, double-blind, multicenter, proof-of-concept trial with adults hospitalized with COVID-19-associated pneumonia and mild to moderate ARDS15. The results established efficacy with a significant proportion of patients remaining alive and free of respiratory failure through day 28 after receiving quisovalimab, most pronounced in patients >60 years of age (76.5% vs. 47.1%, respectively; P = 0.042). These results and animal models validated LIGHT as a target for non-COVID inflammatory conditions, clinical trials ongoing in asthma (NCT05288504)12. - 2021-current: Principal Investigator, Avalo Therapeutics – Sanford Burnham Prebys collaboration
Bioengineered a first-in-class checkpoint agonist targeting BTLA immune checkpoint16 in preclinical development - 2019-current: Director and Principal Investigator, Fair Journey Biologics – Sanford Burnham Prebys collaboration
Immunotherapy for TNBC and PANC, in preclinical development - 2015-2022: Director and Lead Principal Investigator, LILLY-Sanford Burnham Prebys Collaboration in Autoimmunity
Collaborative research partnership with Eli Lilly involved target discovery and therapeutic development directed at immune regulators for autoimmune diseases. The collaboration produced three novel biologics currently in Phase I/2 trials (NCT03933943). The collaboration included a target discovery platform for T cell effector memory and NK cell immunomodulators. - 2015-2020: Lead Principal Investigator, Sanford Burnham Prebys – Capella Biosciences collaboration
Created a fully human mAb specific for membrane LIGHT (CBS001); phase I initiated (NCT05323110). - 2016-2020: Lead Scientific Investigator, Boehringer Ingelheim – Sanford Burnham Prebys Collaboration
Target discovery collaboration in inflammatory and fibrotic diseases6 - 2012-2014: Pfizer Innovation Center, Principal Investigator Bioengineering TNFR Superfamily in Autoimmune disease
- 1. Browning, J.L. et al. Lymphotoxin beta, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell 72,847-856 (1993).
- 2. Crowe, P.D. et al. A lymphotoxin-beta-specific receptor. Science 264,707-710 (1994).
- 3. Mauri, D.N. et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity 8,21-30 (1998).
- 4. Ward-Kavanagh, L.K., Lin, W.W., Sedy, J.R. & Ware, C.F. The TNF Receptor Superfamily in Co-stimulating and Co-inhibitory Responses. Immunity 44,1005-1019 (2016).
- 5. Schneider, K. et al. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell Host Microbe 3,67-76 (2008).
- 6. Virgen-Slane, R. et al. Cutting Edge: The RNA-Binding Protein Ewing Sarcoma Is a Novel Modulator of Lymphotoxin beta Receptor Signaling. J Immunol 204,1085-1090 (2020).
- 7. Sedy, J.R. et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol 6,90-98 (2005).
- 8. Mintz, M.A. et al. The HVEM-BTLA Axis Restrains T Cell Help to Germinal Center B Cells and Functions as a Cell-Extrinsic Suppressor in Lymphomagenesis. Immunity 51,310-323 e317 (2019).
- 9. Stienne, C. et al. Btla signaling in conventional and regulatory lymphocytes coordinately tempers humoral immunity in the intestinal mucosa. Cell reports 38,110553 (2022).
- 10. Sedy, J.R. & Ramezani-Rad, P. HVEM network signaling in cancer. Adv Cancer Res 142,145-186 (2019).
- 11. Croft, M., Benedict, C.A. & Ware, C.F. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov 12,147-168 (2013).
- 12. Ware, C.F., Croft, M. & Neil, G.A. Realigning the LIGHT signaling network to control dysregulated inflammation. J Exp Med 219 (2022).
- 13. Zhang, M., Perrin, L. & Pardo, P. A Randomized Phase 1 Study to Assess the Safety and Pharmacokinetics of the Subcutaneously Injected Anti-LIGHT Antibody, SAR252067. Clin Pharmacol Drug Dev 6,292-301 (2017).
- 14. Perlin, D.S. et al. Levels of the TNF-Related Cytokine LIGHT Increase in Hospitalized COVID-19 Patients with Cytokine Release Syndrome and ARDS. mSphere 5 (2020).
- 15. Perlin, D.S. et al. Randomized, double-blind, controlled trial of human anti-LIGHT monoclonal antibody in COVID-19 acute respiratory distress syndrome. J Clin Invest 132 (2022).
- 16. Sedy, J.R. et al. A herpesvirus entry mediator mutein with selective agonist action for the inhibitory receptor B and T lymphocyte attenuator. J Biol Chem 292,21060-21070 (2017).
 Mar 25, 2025
Mar 25, 2025Engineering antibodies with a novel fusion protein
Mar 25, 2025Fusing two immune system proteins leads to a new method of generating antibodies and may advance drug discovery.
 Nov 19, 2024
Nov 19, 2024Protein superfamily crucial to the immune system experiences Broadway-style revival
Nov 19, 2024San Diego scientists note a resurgence of interest in research on protein family to find new drug candidates.
 Jun 1, 2023
Jun 1, 2023Pumping the brakes on autoimmune disease
Jun 1, 2023New study describes the science behind an autoimmune disease treatment in a Phase 2 clinical trial Researchers at Sanford Burnham…
 Nov 2, 2022
Nov 2, 2022Seeing the immune system in full color
Nov 2, 2022A new flow cytometer at the Institute will help researchers study the immune system with unprecedented resolution and speed. The…
 Apr 6, 2022
Apr 6, 2022How our immune system controls gut microbes
Apr 6, 2022And how this relationship could help fight autoimmune diseases
 Jan 20, 2022
Jan 20, 2022New COVID-19 drug passes phase 2 clinical trial
Jan 20, 2022The new treatment, developed by Avalo Therapeutics with Sanford Burnham Prebys researchers, can mitigate lung damage and improve survival in…
Pamela Itkin-Ansari earned her PhD in Biomedical Sciences from the University of California, San Diego, in 1999. She received postdoctoral training focused on diabetes at that same organization. In 2003, Dr. Itkin-Ansari was appointed Assistant Professor in the Department of Pediatrics, UC San Diego. She moved her laboratory to Sanford Burnham Prebys in 2005.
Funding Awards and Collaborative Grants
California Institute for Regenerative Medicine (CIRM)
Falk Foundation
The Hartwell Foundation
The Hirshberg Foundation
Juvenile Diabetes Research Foundation (JDRF)
National Institutes of Health
Select Honors and Recognition
2013-2014: Outstanding Faculty Mentor Award, UCSD
2011-2014: Hartwell Foundation Biomedical Research Award
2011: Invited Speaker, TEDx
2008-2013: Board of Directors, JDRF San Diego
2008: Health Hero Leadership Award, Combined Health Agencies of San Diego
Other Affiliations
2012-current: Islet Society
2010-current: ASGCT
2008-current: Board of Directors, JDRF San Diego chapter
2008-2013: JDRF board of directors, San Diego
2007-current: American Association for Cancer Research
2007-current: American Diabetes Association
2007-current: American Pediatric Society/Society for Pediatric Research
2006-current: AAAS
Related Disease
Cancer, Diabetes – General, Gastric Cancer, Monogenic Diabetes, Pancreatic Cancer, Type 1 Diabetes, Type 2 Diabetes
Dr. Itkin-Ansari’s research is directed toward understanding diseases of the human pancreas.
Pamela Itkin-Ansari’s Research Report
Diabetes
Areas of focus are: 1) developing a cell-based therapy for diabetes that does not require immunosuppression, and 2) identifying proteins required for proper insulin production and processing.
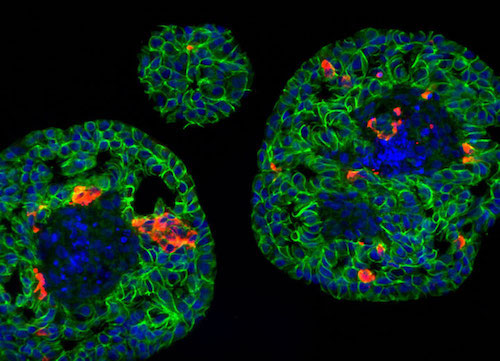
Islet clusters in the developing pancreas
Pancreatic Cancer
The lab determined how dysregulation of specific transcription factors triggers pathogenic cell cycle entry in normal pancreatic cells. This master signaling pathway controlling pancreatic cancer cell growth is yielding potential targets for drug discovery.
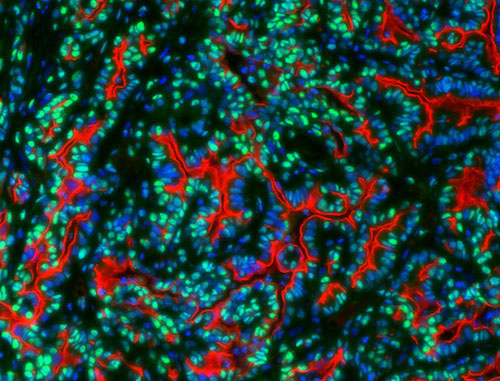
Id3 (green) in human pancreatic cancer
 May 27, 2025
May 27, 2025Scientists and podcasters
May 27, 2025Sanford Burnham Prebys scientists bring dramatic stories of scientific achievement to life.
 May 22, 2024
May 22, 2024Pancreatic cancer symposium celebrates 10th anniversary in San Diego
May 22, 2024The 2024 PancWest Symposium brought more than 120 scientists to the Sanford Burnham Prebys campus in San Diego to discuss…
 Mar 28, 2023
Mar 28, 2023Behind the scenes at Sanford Burnham Prebys’ Cancer Center
Mar 28, 2023Cancer Center open house connects San Diego community with scientists working toward cancer cures
 Oct 26, 2022
Oct 26, 2022Research unlocks the circuitry of diabetes
Oct 26, 2022Research led by Pamela-Itkin-Ansari, PhD, and Randal Kaufman PhD, has mapped out a network of biochemical interactions that help special…
 May 27, 2020
May 27, 2020First map of proinsulin’s “social network” reveals new drug target for type 2 diabetes
May 27, 2020Study reveals previously unknown protein that helps proinsulin fold and opens new avenues for diabetes research Scientists at Sanford Burnham…
 Jan 29, 2016
Jan 29, 2016SBP supports opening of stem cell exhibit at the Reuben H. Fleet Science Center
Jan 29, 2016Pamela Itkin-Ansari, PhD, adjunct assistant professor in the Development, Aging, and Regeneration Program at SBP, participated in the grand opening…
Dr. Dong received his Biology Bachelor of Science degree in 1996 from the University of California, Irvine, where he was involved in molecular evolution and limb regeneration research. He earned his PhD in Cell and Molecular Biology at the University of Wisconsin, Madison in 2002, investigating cell/tissue identity master regulatory genes. His postdoctoral research at the University of California, San Francisco was focused on developmental genetics of the liver and pancreas.
Dr. Dong was recruited as an Assistant Professor to Sanford Burnham Prebys in 2008. He is a recipient of the NIH Director’s New Innovator Award Award and the W. M. Keck Foundation Award, which funds the development of in vivo lineage reprogramming technologies to generate replacement cells and organs directly within a living vertebrate.
Education
BS, Biology, University of California, Irvine
PhD, Cell & Molecular Biology, University of Wisconsin, Madison
Postdoctoral Fellow, Genetics and Development, University of California, San Francisco
Related Disease
Alagille Syndrome, Congenital Diseases, Degenerative Diseases, Liver Diseases, Monogenic Diabetes, Pancreas Diseases, Type 1 Diabetes, Type 2 Diabetes
Phenomena or Processes
Aging, Human Evolution, Organogenesis, Regenerative Medicine
Anatomical Systems and Sites
Developmental Biology, Synthetic Cell Biology
Techniques and Technologies
Developmental Genetics, Disease Genetics
Our objective is to uncover fundamental insight into basic and biomedical science through rigorous investigation of the genetic mechanisms governing organogenesis and diseases. We have discovered multiple genes critical for generating liver and pancreas cells and have created novel animal models for diseases such as diabetes and Alagille Syndrome. These unique experimental models have been yielded mechanistic insight and potential new therapeutic avenues. Further, we have demonstrated for the first time that a cell’s identity can be reprogrammed to convert into a completely unrelated lineage, without their removal from the body (in vivo) and without passage through a stem cell intermediate. This in vivo lineage reprogramming breakthrough may lead to a vast new and safer source of replacement cells for degenerative diseases and injuries. Ultimately, we aim to develop genetic technologies to improve human health and advance human biology.

Kashton (diagnosed with Alagille Syndrome), his mother Shauna, and Professor Dong observing zebrafish
with a mutation in jagged, the gene affected in his disease.
 Jan 9, 2023
Jan 9, 2023New Hope Against ‘Incurable’ Liver Disease That Kills Children
Jan 9, 2023Alagille syndrome is caused by a mutation that prevents the formation and regeneration of bile ducts in the liver. About…
 Jan 4, 2023
Jan 4, 2023Incurable liver disease may prove curable
Jan 4, 2023Research from Sanford Burnham Prebys has found a drug that can spur liver regeneration in Alagille syndrome.
 Jun 7, 2022
Jun 7, 2022Alagille syndrome: a promising therapy races towards clinical trials
Jun 7, 2022Recent breakthroughs from Professor Duc Dong and his team bring great promise to the Alagille community and are also cause…
 Mar 30, 2022
Mar 30, 2022From zebra fish to bacteria, Diabetes Research Connection celebrates a decade funding novel ideas
Mar 30, 2022San Diego group has provided more than $2 million to early-career scientists across the country conducting maverick research on Type 1 diabetes
 Oct 11, 2021
Oct 11, 2021New stem cell identified by Sanford Burnham Prebys researchers offers hope to people with rare liver disease
Oct 11, 2021Researchers have discovered a new source of stem cells just outside the liver that could help treat people living with…
 Mar 25, 2019
Mar 25, 2019Inspiring future scientists at the STEM EXPO
Mar 25, 2019Armed with wiggly worms and striped zebrafish, on Saturday, March 2, more than 20 volunteers from Sanford Burnham Prebys helped…