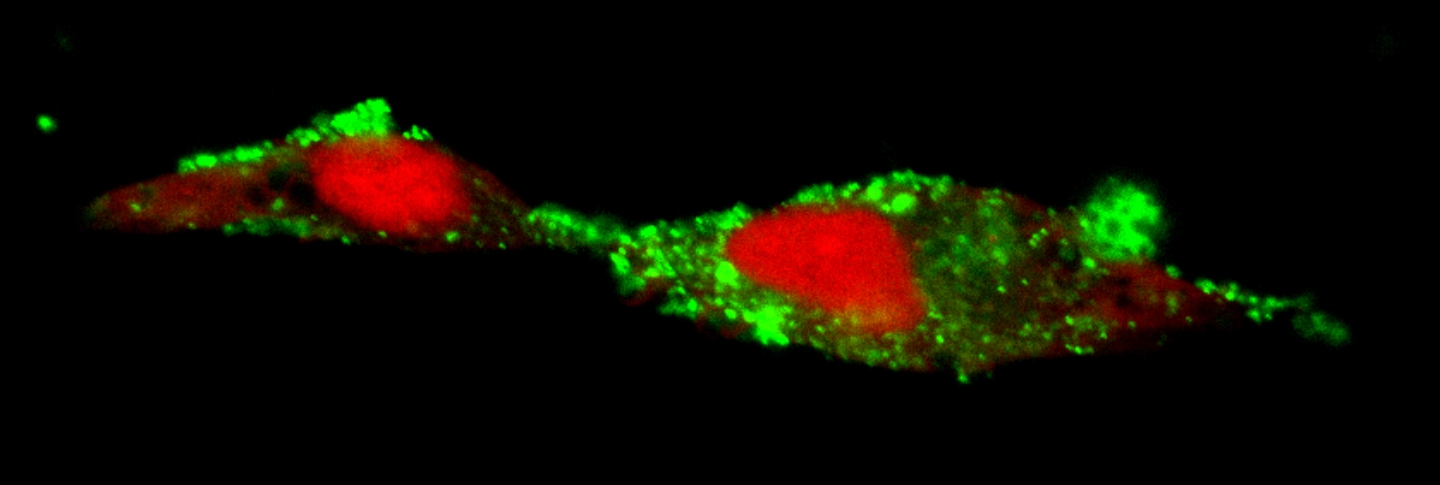
In Alzheimer’s disease, the space around brain cells becomes clogged with toxic clumps of protein called amyloid. The problem isn’t just that amyloid is generated—that happens in all aging brains—it’s that there’s more of it than the brain’s garbage-scavenging cells, called microglia, can clear out. The lingering amyloid causes neurons to break down, creating a bigger mess for the already overworked microglia to eliminate.
Recent research could lead to a way to turn up the activity of microglia, which should slow the advance of Alzheimer’s. Two related papers with contributions from Huaxi Xu, PhD, the Jeanne and Gary Herberger Chair of Neuroscience and Aging Research at Sanford Burnham Prebys Medical Discovery Institute (SBP), show that a protein called TREM2 helps microglia survive and respond more strongly to damaging material like amyloid and cell debris.
“These results suggest that activating TREM2 could be a viable future treatment strategy for Alzheimer’s,” says Xu. “TREM2 has recently become a hot topic since mutations in the gene have been strongly linked to a greater risk for Alzheimer’s. This work shows that TREM2 is important not just in the relatively small number of patients carrying mutations, but potentially in all of Alzheimer’s.”
TREM2 is a receptor on tissue-resident immune cells, including microglia. TREM2 is activated by fatty molecules released from damaged cells, which stick to amyloid.
The two papers identify the functions of two forms of TREM2—the receptor form that sits on the surface of microglia, and a soluble fragment form that’s released into the space surrounding cells in the brain. Both studies were led by Guojun Bu, PhD, professor at the Mayo Clinic. The first, published in the Journal of Neuroscience, shows that the receptor form of TREM2 is required for microglia to live as long as they normally do. The second, in the Journal of Experimental Medicine, found that the soluble TREM2 fragment also supports microglial survival and turns on their inflammatory response—which in turn supports their ability to remove toxic amyloid clumps.
“The finding that soluble TREM2 has an important function supports its use as a biomarker for Alzheimer’s,” adds Xu. “Levels of soluble TREM2 increase in cerebrospinal fluid before severe symptoms appear, so measuring its levels could aid early diagnosis, which is crucial for effective treatment.”
“We’re now searching for activators of TREM2 in collaboration with drug discovery specialists here at SBP and with support from the Tanz Initiative,” Xu comments. “We’re also looking at whether enhancing TREM2 function lowers levels of amyloid in models of Alzheimer’s.”