Just about any of the 700,000-plus people in the U.S. who have Crohn’s disease, a chronic condition in which portions of the intestine are inflamed, can tell you that better treatments are urgently needed. Even with medication, most experience flare-ups that keep them away from work or school for days or weeks at a time with diarrhea and severe abdominal pain.
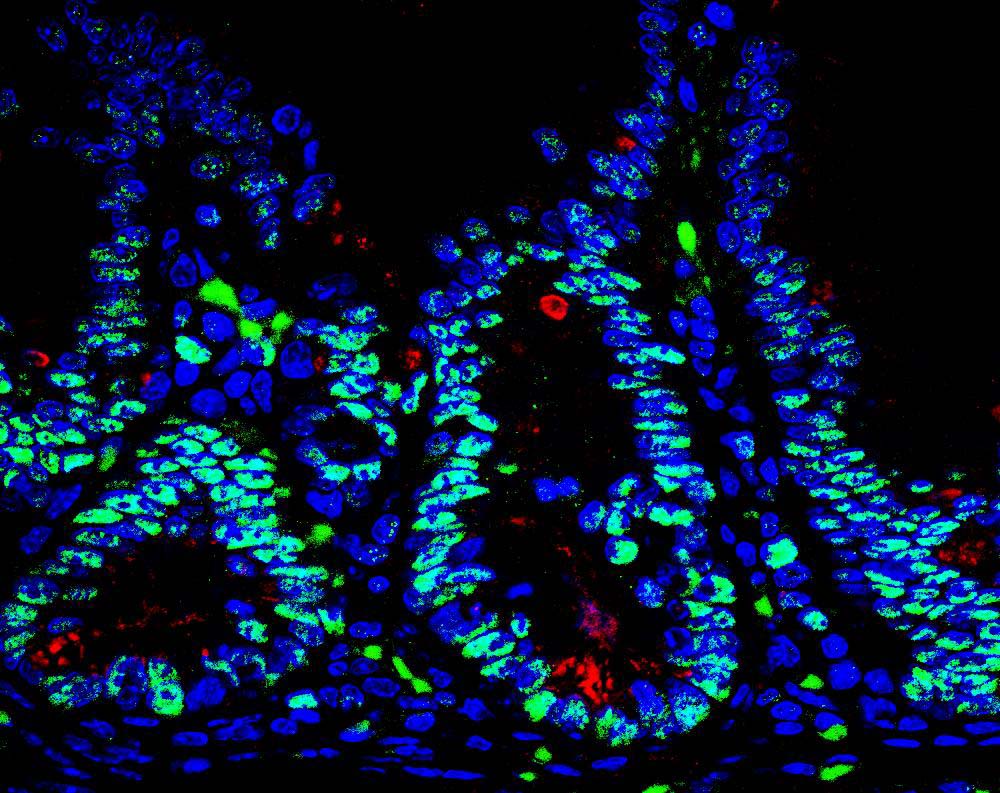
A soon-to-commence clinical trial could offer Crohn’s patients new hope. The trial will test a drug made possible by a discovery from the lab of Carl Ware, PhD, professor and director of the Infectious and Inflammatory Diseases Center at Sanford Burnham Prebys Medical Discovery Institute (SBP). Almost two decades ago, his team identified an immune-regulating protein called LIGHT, and went on to show that it promotes intestinal inflammation. The new drug is a protein that prevents LIGHT from binding its target receptor on T cells, the immune cells that drive inflammation in Crohn’s disease.
“We’re thrilled to see our research through to a clinical trial,” said Ware. “We’ve focused on patients with a rare mutation that causes especially severe, early-onset disease. If the drug proves beneficial to those patients, we expect to pursue a larger trial in patients with typical Crohn’s.”
The trial will test the LIGHT-blocking drug in children with Crohn’s disease who carry a mutation in the gene for a protein called DcR3, which normally limits the amount of LIGHT and two other related pro-inflammatory molecules. The genomics company Medgenics will conduct the clinical trial with physician-scientist Robert Baldassano, MD, at the Children’s Hospital of Philadelphia.
Ware’s lab will be involved in fundamental research aspects of the study, developing assays to measure levels of LIGHT in patient plasma. In parallel, his team will be studying how the drug affects the microbiome in animal models.
“We are also applying what we’ve learned about LIGHT and the receptors it interacts with to other autoimmune diseases,” said John Sedy, PhD, research assistant professor in Ware’s group. “We’re working towards treatments that modulate the immune system in different ways.”