Integrins are indispensable cell-surface proteins that form bridges with the surrounding protein matrix and other cells. They also transmit the biochemical and mechanical signals that regulate essential cellular processes, such as proliferation, differentiation, migration and cell death. Understanding how integrins become activated is important for developing ways to modulate their function, which is relevant to many disease processes, including cancer, autoimmunity, and fibrosis (hardening of tissue).
The laboratory of Dorit Hanein, PhD, in collaboration with Niels Volkmann, PhD, both professors in SBP’s Bioinformatics and Structural Biology Program, have made an important contribution in this area. Their recent study fundamentally changes our understanding of how integrins are activated—instead of a binary one-step process, it turns out to be a much more nuanced process involving multiple states.
The details
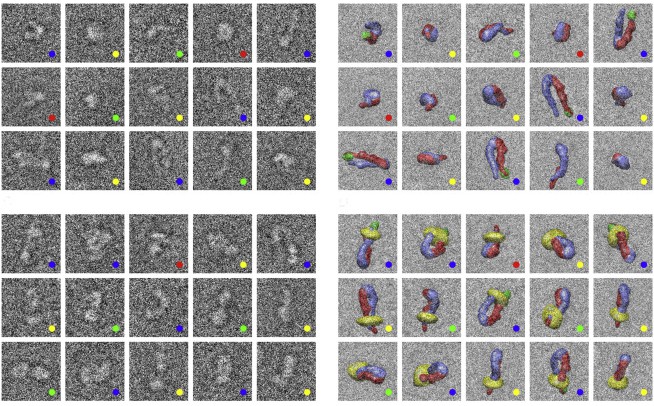
Publishing in the Biophysical Journal, the team used cryo electron microscopy (cryoEM) and advanced computational methods to determine the three-dimensional structures of full-length human integrin, αIIbβ3, which mediates blood clotting. CryoEM allows scientists to look at how a protein works in a near-physiological environment—surrounded by water and interacting with other proteins.
“Because cryoEM captures multiple states, we were able to show that the activation of integrins is much more complex than we thought—they exist in an equilibrium of conformations between bent and upright, and binding to their partners makes them more likely to straighten. Also, the matrix-binding domain is accessible even in the bent form, making it clear how integrins can be initially activated by extracellular signals,” explained Hanein.
Until now, the model for integrin activation was based on structures generated using other techniques that have value, but significant drawbacks as well. For example, X-ray crystallography constrained the protein to a highly compact and bent conformation, which implied an inactive configuration. Another type of electron microscopy revealed an upright conformation, assumed to be the active form that’s triggered by a one-step switch.
What the results mean
The new model aligns better with integrins’ ability to integrate multiple signals to control key cell processes. Instead of one binding event turning the integrin all the way on, each additional signal shifts it towards the upright form, which transmits mechanical signals more easily.
This refinement of the model is critical for designing and interpreting experiments involving integrins. According to Volkmann, “To do translational research, you have to understand the language, and in this case the language is structure.”
The paper is available online here.