Cynthia Lebeaupin, PhD was recently awarded the 2022 Fishman Fund Fellowship, a postdoctoral award unique to Sanford Burnham Prebys.
The award provides a boosted stipend to exceptional postdocs from the Institute who have a demonstrated research track record and whose work shows significant potential for future breakthroughs.
“It’s an honor to have been selected for such a prestigious award from the Institute, says Lebeaupin, who works in the lab of Randal J. Kaufman, PhD “The resources and people at Sanford Burnham Prebys are incredible and I’m happy to be able to continue my research here.”
Sanford Burnham Prebys introduced the Fishman Fund Awards in 2001 to honor of the Institute’s founders, Dr. William and Lillian Fishman. The fund was established by Reena Horowitz and the late Mary Bradley, longtime supporters of the Institute.
“The Fishmans created an Institute that fosters a collaborative, inspirational atmosphere for postdoc students,” said Horowitz at the 2021 Fishman Fund Awards. “The Fishmans understood that support for new science is a brilliant research investment.”
Lebeaupin has been at the Institute since 2018, and this is not her first honor from the Fishman Fund. In 2021, she was awarded a Fishman Fund Career Development Award, a smaller prize offered to several postdocs each year. She also completed an internship at the Institute’s former Lake Nona campus in 2014.
“I’ve had an affinity for Sanford Burnham Prebys for a long time,” says Lebeaupin. “I knew once I met Dr. Kaufman and everybody on campus that this was the best place to complete my postdoc.”
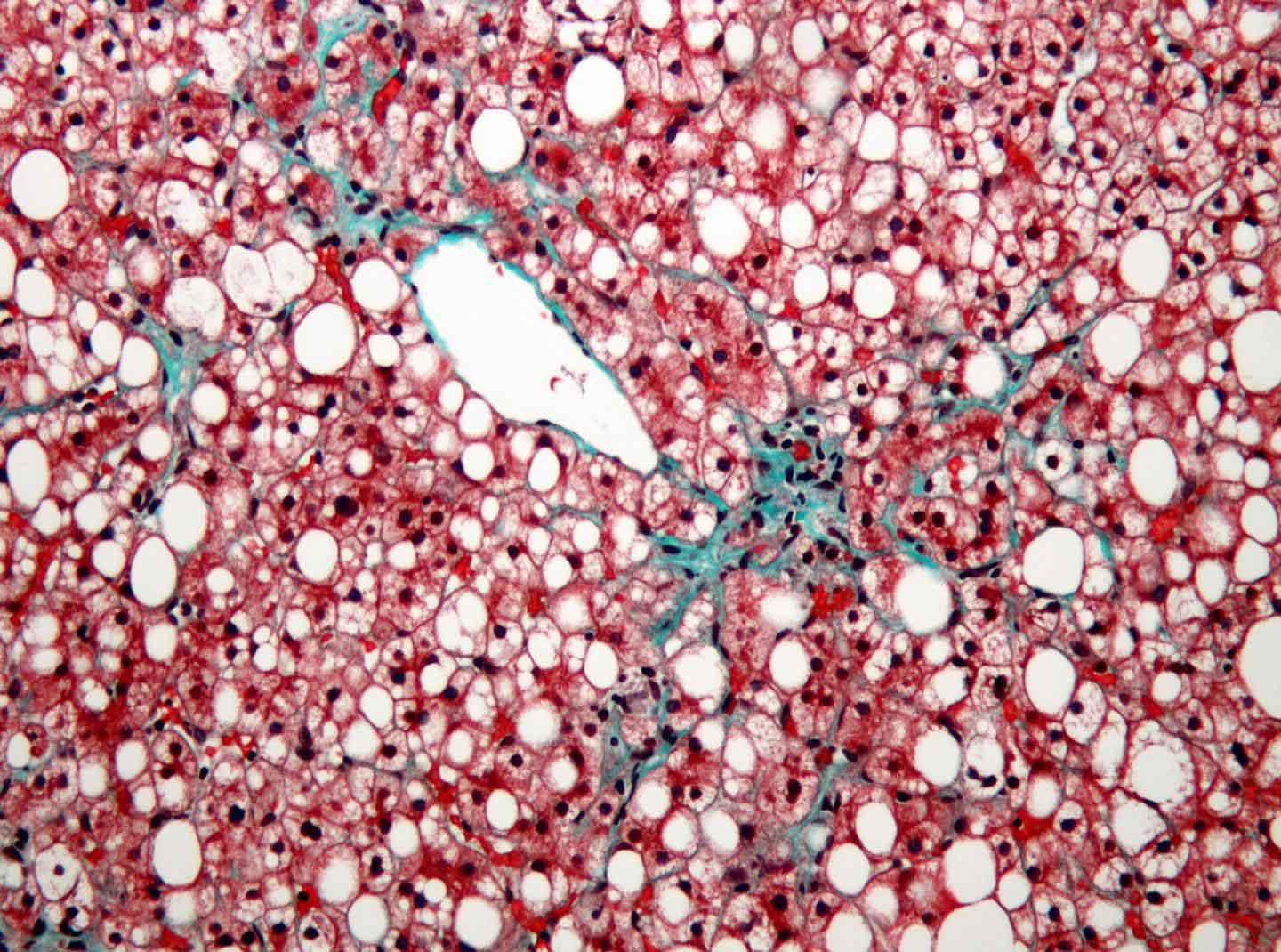
Lebeaupin’s research focuses on a growing and pressing problem in medicine – liver cancer. One of the major risk factors for developing liver cancer is fat accumulation in the liver, known as fatty liver disease. Increases in obesity rates over the last several decades have led to a dramatic increase in fatty liver disease.
Fatty liver disease is increasing at an alarming rate, and unfortunately, it’s here to stay,” says Lebeaupin. “My research is figuring out how fatty liver disease progresses to liver cancer, so we can use this knowledge to help prevent it.”
In particular, Lebeaupin is working on exploring how cells respond to fatty liver disease over time. She discovered that a molecule that helps liver cells protect themselves from short-term stress can promote cancer in the long-term. She has now moved into studying the system in human tissues.
“This research is exciting because we aim to translate our discoveries from the bench to the bedside,” says Lebeaupin. “What I hope to do in the future is use new technologies on liver samples from patients so we can identify what’s actually going on in liver diseases.”