Scientists at Sanford-Burnham Medical Research Institute (Sanford-Burnham) have identified how an enzyme called PKCζ suppresses prostate tumor formation. The finding, which also describes a molecular chain of events that controls cell growth and metastasis, could lead to novel ways to control disease progression.
Working in close collaboration, the labs of Maria T. Diaz-Meco, PhD, and Jorge Moscat, PhD, found that PKCζ controls the activation of a pro-tumor gene called c-Myc. Normally, PKCζ's alteration keeps c-Myc in check. But PKCζ levels are low in prostate and other cancers, leaving c-Myc free to enhance cell growth and metastasis. This study, published April 1 in the Proceedings of the National Academy of Sciences, suggests that restoring PKCζ could provide a new approach to treating prostate cancer.
How PKCζ acts as a prostate tumor suppressor
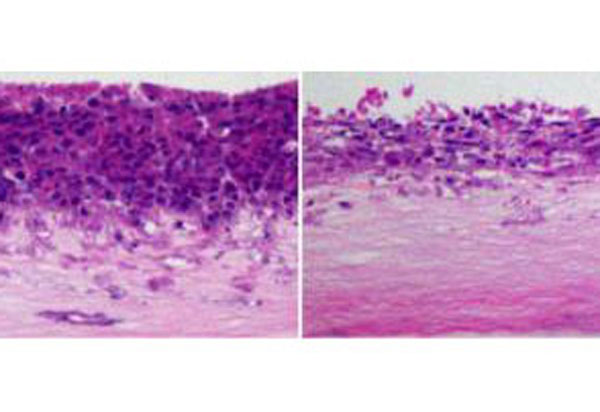
Previous studies suggested that PKCζ might act as a tumor suppressor--but that wasn't clear in the case of prostate cancer. In their study, the team learned of PKCζ's role after genetically engineering mice so they lacked the enzyme altogether.
"In this study, we assessed the role of PKCζ in prostate cancer, and for the first time we used a knockout mouse for PKCζ to demonstrate that it's actually a tumor suppressor," Diaz-Meco said. "But, I think the major advance in this paper is that we found out how PKCζ is a tumor suppressor in prostate cancer."
In their study, the researchers found that PKCζ suppresses tumors in cooperation with a gene called PTEN. PTEN has been long known to act as a tumor suppressor, and it's also well-established that its mutated form is common in prostate cancer.
But the loss of normal PTEN function alone doesn't lead to aggressive prostate cancer. According to this study, the loss of PKCζ and the resulting over-active c-Myc are also needed for aggressive prostate cancer to develop.
Potential approaches toward attacking prostate cancer may in the future involve activating PKCζ through gene therapy, or dealing with its inaction downstream--perhaps by finding another way to inhibit c-Myc in the absence of PKCζ.