Elena Pasquale earned her PhD in biology from the University of Parma, Italy. She did postdoctoral work at Cornell University, after which she was appointed Research Assistant Professor at University of Parma. Following a second postdoctoral training period at the University of California in San Diego, Dr. Pasquale was appointed Assistant Research Biologist at that institution. Dr. Pasquale was recruited to Sanford Burnham Prebys in 1990.
Related Disease
Cancer, Neurodegenerative and Neuromuscular Diseases, Skin Cancer and Melanoma
Cancer, Neurodegenerative and Neuromuscular Diseases
Receptor tyrosine kinases of the Eph family and their ligands, the ephrins, represent an important cell communication system that controls a vast array physiological and disease processes. For example, Eph receptors and ephrins take part in the formation of blood vessels, including the blood vessels that feed tumors, and regulate the malignant properties of cancer cells and their interplay with the tumor microenvironment. They also regulate the formation, plasticity and regeneration of neuronal circuits as well as neurodegenerative processes such as those occurring in amyotrophic lateral sclerosis (ALS) and Alzheimer’s disease. The signal transduction mechanisms of Eph receptors are intriguing, and complex, because these receptors engage in multiple modes of signaling. Binding to ephrin ligands on the surface of neighboring cells induces canonical signaling involving receptor clustering, autophosphorylation on tyrosine residues, and kinase activity-dependent downstream signaling. Binding to the Eph receptors can also cause the ephrins, which have a cytoplasmic domain or a GPI-anchor, to transmit signals. This leads to bidirectional signals emanating from Eph receptor-ephrin complexes positioned at sites of cell-cell contact. In addition, at least some Eph receptors can also signal through non-canonical mechanisms that are independent of ligand binding and kinase activity, for example through interplay with other receptor tyrosine kinase families and with serine/threonine kinases.
Our research investigates Eph receptor signaling activities in order to understand their role in normal physiology and in pathological conditions such as cancer and neurodegenerative disorders. This knowledge is useful for the development of disease treatments based on modulating Eph receptor/ephrin activities. Ongoing efforts in our laboratory also focus on the development of agents targeting Eph receptors for research and translational applications.
Elena Pasquale’s Research Report
We discovered several Eph receptors and ephrins, and research in our laboratory is dedicated to the characterization of Eph receptor signal transduction mechanisms and biological functions using biochemical, mass spectrometry, molecular biology and cell biology approaches in conjunction with animal models. We have identified tyrosine and serine/threonine phosphorylation sites of Eph receptors and ephrins using mass spectrometry and investigated the signaling role of these phosphorylation sites. For example, our past work showed that two conserved tyrosine phosphorylation sites in the juxtamembrane segment of the Eph receptors not only mediate association with binding partners but also regulate receptor kinase activity. We also found that the SRC and ABL non-receptor tyrosine kinases and the SHEP1 scaffolding protein are binding partners of the Eph receptors, and we identified signaling connections between Eph receptors and integrins. We also found that EphA4 is highly expressed in the adult brain, where it regulates synaptic connections. More recent work in our laboratory focuses on elucidating signaling pathways that mediate the activities of Eph receptors in cancer cells.
Tumor Suppression and Tumor Promotion by Eph Receptors
Many Eph receptors are highly expressed in tumors, but their role in cancer is incompletely understood and likely depends on the cellular context. Certain Eph receptors and ephrins promote tumor angiogenesis. We showed that the EphA2 receptor is upregulated in the tumor vasculature together with the ephrin-A1 ligand, which suggested a role in tumor angiogenesis that is now well established. We also found that the EphB4 receptor expressed on the surface of breast cancer cells can promote tumor xenograft growth by enhancing blood vessel formation through interactions with its preferred ligand, ephrin-B2, present in tumor endothelial cells. Additional intriguing roles for the Eph receptors in cancer progression have also emerged. We found that canonical signaling by the EphB4 receptor is low in breast cancer cells and that ephrin-induced stimulation of EphB4 kinase activity inhibits breast cancer cell malignancy in culture and tumor growth in vivo (Figure 1A) through inhibition of the CRK proto-oncogene. More recently, we elucidated an additional mechanism of tumor suppression mediated by canonical ephrin-induced EphA2 signaling (Figure 1A), which leads to inhibition of the AKT-mTORC1 oncogenic pathway through interplay of EphA2 with a phosphatase that dephosphorylates the AKT serine/threonine kinase.

Figure 1. Dual activities of Eph Receptors in Cancer Cells. (A) Eph receptor-ephrin binding at cell-cell contact sites results in the dimerization/clustering of Eph receptor-ephrin complexes, and initiation of canonical signals through the receptor cytoplasmic domain. Signals through the ephrins can also be generated. Tyrosine phosphorylation sites (yellow circles) promote Eph kinase activity and also provide binding sites for signaling proteins containing SH2 domains. Other effectors also mediate Eph signals, including PDZ domain-containing proteins. The Eph receptor domains are indicated on the left; LBD, ligand-binding domain. (B) Eph receptors can potentiate the oncogenic effects of other receptors. These activities are independent of ephrin binding and/or kinase activity and their mechanism is not well understood but in some cases depends on Eph receptor phosphorylation on serine/threonine residues (red circle).
There is also evidence that some Eph receptors can increase cancer cell malignancy through non-canonical ephrin-independent and/or kinase-independent signaling activities (Figure 1B), which is the subject of ongoing work. These tumor promoting activities include inducing invasiveness and metastasis, epithelial-to-mesenchymal transition, stem cell-like features and drug resistance.
Eph Receptor Mutations in Cancer
The Eph receptors are frequently mutated in many types of cancer. In particular, genome-wide sequencing studies have detected somatic mutations in one or more Eph receptors in 25%-45% of melanomas, 15%-45% of lung cancers, 25-40% of colorectal cancers and 12%-25% of head and neck and uterine cancers (Figure 2), but very limited information is available on the effects of the mutations. Studies by ours and other groups have shown that a number of EphA2 and EphA3 mutations inactivate Eph receptor canonical signaling by disrupting ephrin binding or kinase activity, consistent with a role of canonical signaling in tumor suppression. Ongoing work in our laboratory focuses on characterizing the functional effects of Eph receptor mutations in cancers such as melanoma, and investigating whether the mutations shift the balance of the Eph receptor signaling activities from tumor suppression to tumor promotion. We are also interested in the interplay of Eph receptor mutations with mutations affecting well-established oncogenes and tumor suppressor genes. Understanding the effects of Eph receptor mutations in cancer cells will help shed light on the role of the Eph receptor/ephrin system in cancer cell transformation, malignant progression and drug resistance.
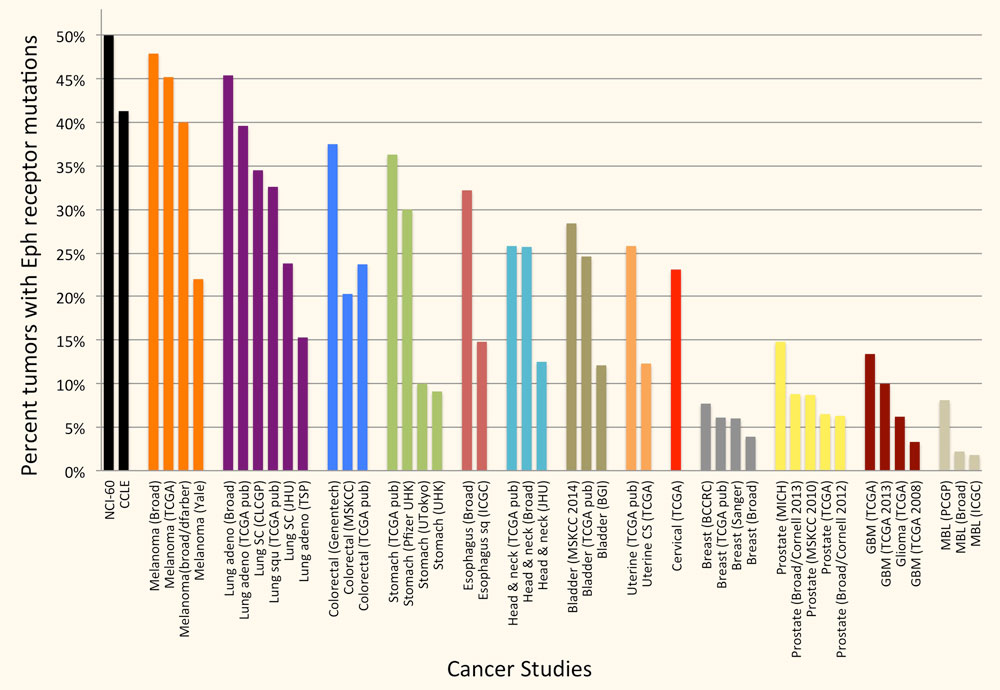
Figure 2. A large percentage of tumor specimens and cell lines harbor one or more Eph receptor mutations. Groups of bars of the same color represent studies of the same cancer type. The cancers with most Eph receptor mutations are shown; other tumor types have fewer or no Eph receptor mutations. The graph is based on data from cBioPortal for Cancer Genomics (www.cbioportal.org).
Peptides Targeting Eph Receptors
We have identified a number of peptides that bind to Eph receptors and inhibit ephrin binding by using phage display approaches. Collaborating groups have elucidated the structural features of several of these peptides in complex with the ligand-binding domain of Eph receptors, demonstrating that the peptides bind to the ephrin-binding pocket in the ligand-binding domain (Figure 3A). Most of the peptides are antagonists, but the peptides targeting EphA2 are agonists that activate receptor signaling and endocytosis similarly to the natural ephrin ligands. Interestingly, some of the identified peptides are highly specific and bind to only one Eph receptor family member. This is unlike the natural ephrin ligands, each of which promiscuously binds to multiple Eph receptors. Thus, Eph receptor-targeting peptides represent valuable pharmacological tools to study the functional importance of specific Eph receptors in tumors and the nervous system. Furthermore, they could be used as leads to develop therapies against cancer and neurological disorders, and to promote neural repair after nervous system injury (Figure 3B). Finally, our peptides have been used by other groups to deliver conjugated imaging agents, drugs and nanoparticles to Eph receptor-positive tumors. Current work focuses on identifying novel Eph receptor-targeting agents (such as peptides and small molecules) as well as improving the existing ones in collaboration with medicinal chemists and structural biologists, and evaluating them in cell culture and in vivo animal models.
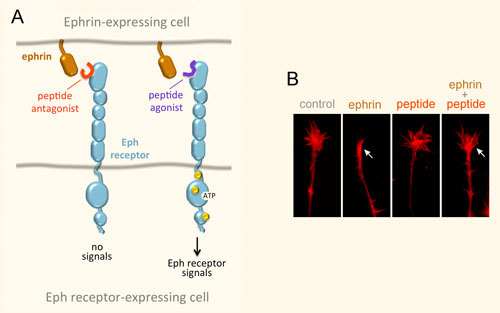
Figure 3. Peptides can target the ephrin-binding pocket of Eph receptors with high affinity and specificity, affecting receptor function. (A) Peptides targeting the Eph receptors can function as antagonists that inhibit ephrin binding and receptor signaling, or in some cases as agonists that mimic the ephrins by activating Eph receptor signaling. Yellow circles indicate tyrosine phosphorylation sites in the activated Eph receptor. (B) An EphA4 peptide antagonist blocks ephrin-induced growth cone collapse in EphA4-expressing axons, suggesting its usefulness for promoting neural repair. The arrow in the second panel marks a growth cone collapsed due to ephrin treatment; the arrow in the fourth panel marks a growth cone that did not collapse following ephrin treatment in the presence of a peptide antagonist.
 Jan 7, 2025
Jan 7, 2025Mutations in protein receptor gene linked to Alzheimer’s disease
Jan 7, 2025New research on four variants in the EPHA1 gene reveals how its genetic typos may contribute to risk of dementia.
 Nov 27, 2023
Nov 27, 2023The “Eph” system may pave the way for novel cancer therapies
Nov 27, 2023Over the past three decades, researchers have been investigating an important cell communication system called the “Eph system,” and the…
 May 13, 2019
May 13, 2019Targeting long-sought EphA2 receptor becomes crystal clear
May 13, 2019Scientists have long sought to target a cellular receptor called EphA2 because of its known role in many disorders, including…
 Jul 31, 2017
Jul 31, 2017SBP researchers awarded Padres Pedal the Cause collaborative grants
Jul 31, 2017Sanford Burnham Prebys Medical Research (SBP) is pleased to announce that it has been awarded five collaborative grants with the…
 May 16, 2017
May 16, 2017What SBP Scientists are Researching to Battle Skin Cancer
May 16, 2017Skin cancer is one of the most common of all cancers, and melanoma accounts for about 1 percent of skin…
 Oct 29, 2015
Oct 29, 2015The Collaboration 4 Cure Alzheimer’s research awards announced at SBP
Oct 29, 2015On October 28, Mayor Kevin Faulconer, County Supervisor Diane Jacob, San Diego philanthropist Darlene Shiley, and Mary Ball, president and…
