The developing nervous system in the eye of a 7-day-old chick embryo.
Image courtesy of Elkhan Yusifov and Martina Schaettin, University of Zurich/Nikon Small World.
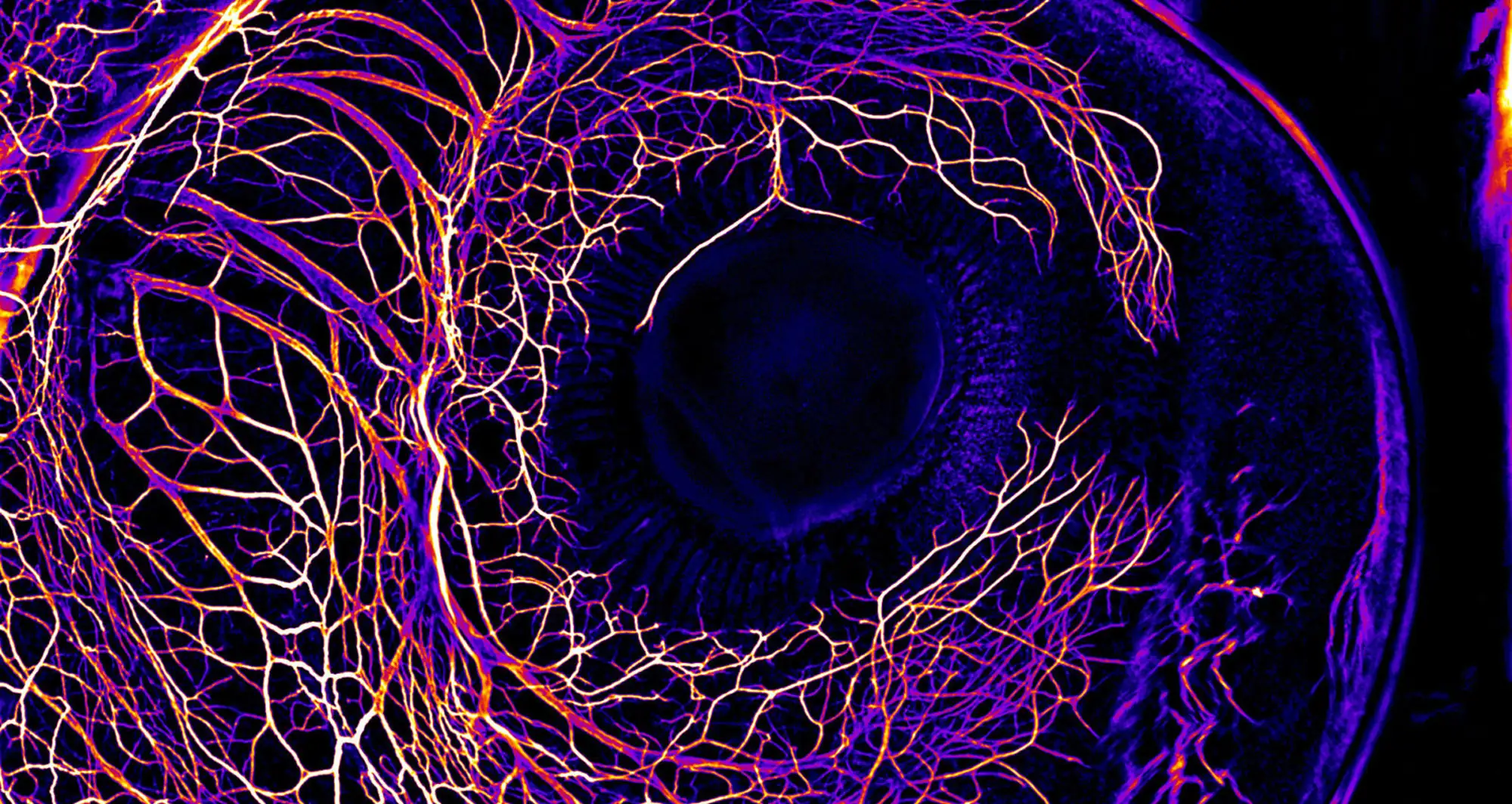
The developing nervous system in the eye of a 7-day-old chick embryo.
Image courtesy of Elkhan Yusifov and Martina Schaettin, University of Zurich/Nikon Small World.

In his latest letter examining the effects and ramifications of the Trump administration’s assault on science, President and CEO David Brenner, MD, of Sanford Burnham Prebys discusses how other countries recruiting US-based scientists to work or start their careers elsewhere, with greater support and scientific freedom.
You can read Dr. Brenner’s letter here, plus earlier letters and other materials related to Research in Crisis.

The abdominal skin of a tick engorged with blood. The ridged construction of the skin allows feeding ticks to expand up to 100 times their body weight.
Image courtesy of Theo Theune, Oost-Souberg, Zeeland, The Netherlands/Nikon Small World.

Lymphatic vascular (cyan) and blood vessels (red) are revealed in this confocal micrograph of a mouse lung. The lymph system collects excess fluid in tissues and returns it to bloodstream.
Image courtesy of Yurin Seo, Mark Looney and Simon Cleary, UCSF/Nikon Small World.
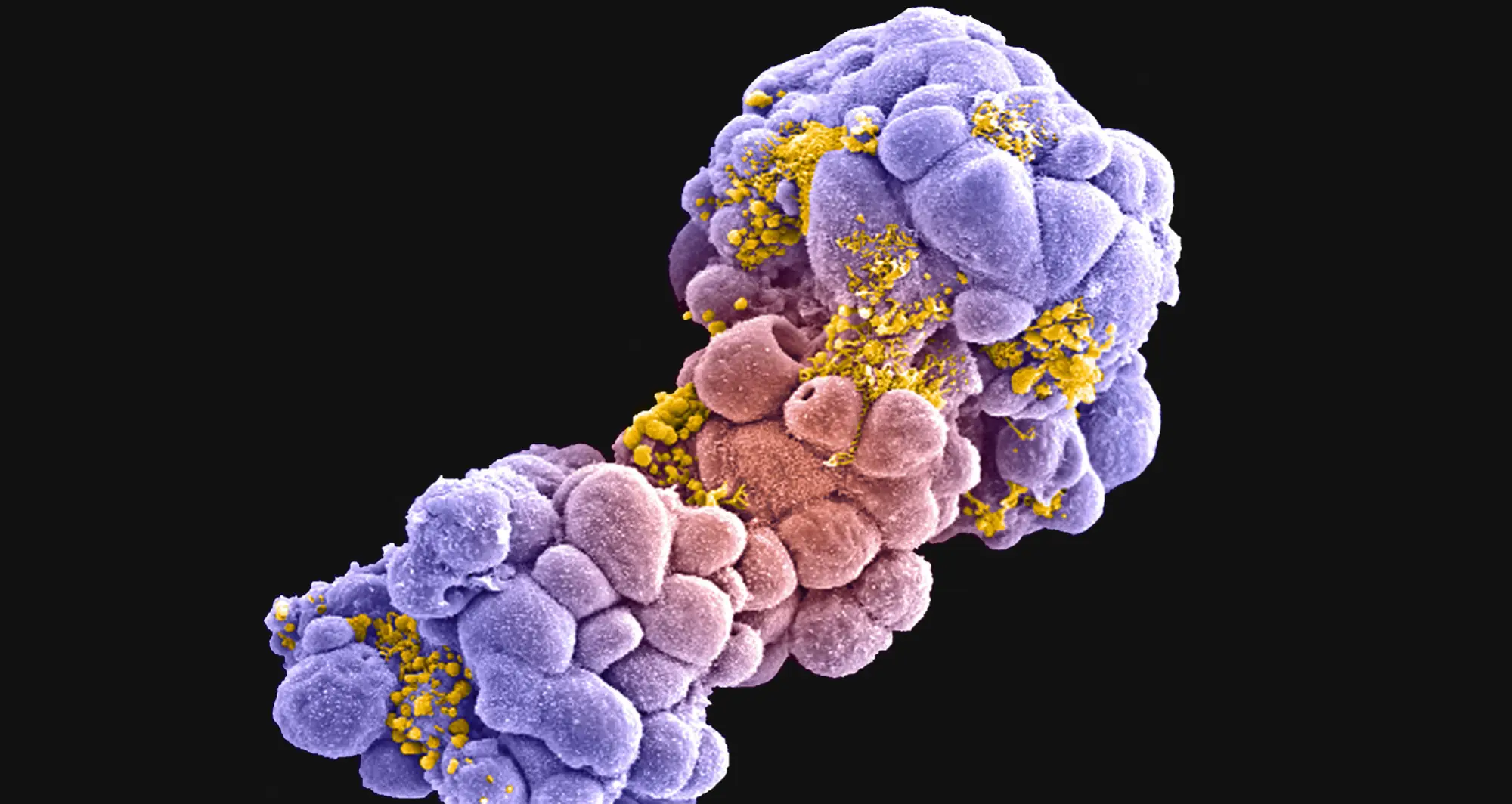
PERCEPTION is the acronym for PERsonalized Single-Cell Expression-Based Planning for Treatments In Oncology, an artificial intelligence-based tool that, in findings first reported last year, was able to predict tumor response to targeted therapy using single-cell datasets.
The work, published in Nature Cancer, is the result of first study author Sanju Sinha, PhD, assistant professor in the Cancer Metabolism and Microenvironment Program at Sanford Burnham Prebys, with senior authors Eytan Ruppin, MD, PhD, and Alejandro Schaffer, PhD, at the National Cancer Institute (NCI), part of the National Institutes of Health, and colleagues.
Recently, the NCI’s Center for Cancer Research highlighted PERCEPTION in its 2024-2025 annual Milestones report.
The researchers said PERCEPTION not only helped predict which anti-cancer drugs are most effective for individual patients, but also tracked the evolution of drug resistance over the course of the disease and treatment — something never before achieved.
“A tumor is a complex and evolving beast. Using single-cell resolution can allow us to tackle both of these challenges, Sinha said when their findings were published. “PERCEPTION allows for the use of rich information within single-cell omics to understand the clonal architecture of the tumor and monitor the emergence of resistance.” (In biology, omics refers to the sum of constituents within a cell.)
“The ability to monitor the emergence of resistance is the most exciting part for me. It has the potential to allow us to adapt to the evolution of cancer cells and even modify our treatment strategy.”
PERCEPTION was previously named among the National Institutes of Health director’s highlights for 2024.

The annual Rising Stars Symposium featured nine doctoral-degree candidates representing the next generation of scientists
Nine doctoral-degree candidates from across the U.S. visited Sanford Burnham Prebys for the fourth annual Rising Stars Symposium, a research meeting and networking opportunity for future postdoctoral researchers.
James Marchant, PhD, a postdoctoral fellow in the lab of Alexandre Colas, PhD, at Sanford Burnham Prebys, opened the meeting by introducing the symposium’s keynote speaker, Mark Mercola, PhD, a professor of Medicine at the Stanford University School of Medicine and a professor in the Stanford Cardiovascular Institute.
“We’re so pleased to have a keynote address from a stem cell biology expert responsible for identifying many of the factors that guide the formation of the heart,” said Marchant. “This also is a homecoming for Mark, who was previously on the faculty here and continues to be an extended family member through collaborations and service on dissertation committees.”
Mercola held a joint appointment as a professor at Sanford Burnham Prebys and the University of California San Diego from 2003-15 before joining Stanford. He began his lecture with a discussion of how his research evolved throughout his career, including the opportunities provided by the discovery of induced pluripotent stem cells. Mercola now focuses on developing treatments for heart failure that target mechanisms rather than symptoms.
“While drugs for heart failure have improved over time, they continue to generally improve heart function by altering conditions such as blood pressure and heart rate rather than target the underlying causes,” said Mercola. “I envision a day when treatment begins to resemble modern cancer therapies that are targeted based on understanding characteristics of tumors.”
Mercola also offered career advice to the Risings Stars. He emphasized the importance of pursuing a long-term vision that can guide how you seek training, apply for funding and build your network. Mercola suggested that this vision needs to be balanced with measurable short-term goals that can show your intermediate progress along the way. He also implored early career scientists not to stay isolated in their own labs.
“Be collaborative, and you’ll find that the work is always more enjoyable and successful when you are part of a team,” said Mercola.
Following the keynote address, four Rising Stars discussed their research projects:
Lukas Chavez, PhD, an associate professor in the Cancer Genome and Epigenetics Program at Sanford Burnham Prebys, offered concluding remarks at the end of the symposium’s first day.
“With their excellent presentations, our Rising Stars have lived up to their name,” said Chavez. “It is exciting and reassuring to see that the next generation of biomedical research is in capable hands.”
David Brenner, MD, the president and chief executive officer of Sanford Burnham Prebys, began the symposium’s second day by sharing an overview of the institute and San Diego’s scientific community.
“There is something special in the intellectual environment here at Sanford Burnham Prebys and our neighboring research institutions,” said Brenner. “If you conduct a postdoctoral fellowship here, you will find it to be a very welcoming, collaborative and interactive ecosystem.”
Sanju Sinha, PhD, an assistant professor in the Cancer Metabolism and Microenvironment Program at Sanford Burnham Prebys, added his reflections on the importance of scientific meetings for early career researchers.
“I place great value on these kinds of symposia,” said Sinha. “I met many of my collaborators at a similar symposium in New York, so I encourage the Rising Stars to take advantage of this opportunity to get to know each other and investigators throughout the institute.”
After the opening remarks, five Rising Stars presented about their research:
In addition to the two days of scientific talks, the Rising Stars toured the Conrad Prebys Center for Chemical Genomics, learned about the institute’s core facilities and shared research resources, networked with institute scientists, and gained a better understanding of postdoctoral opportunities at Sanford Burnham Prebys.
The 2025 Rising Star Symposium was sponsored by the NCI-designated Cancer Center and was planned collaboratively by the institute’s Workforce Engagement and Belonging Council and planning and selection committees.
The planners expressed their gratitude to everyone who contributed to this institute-wide effort, including session moderators and facilitators, Cancer Center administration, the Research Administrative Services team, the Communications team and many volunteers.
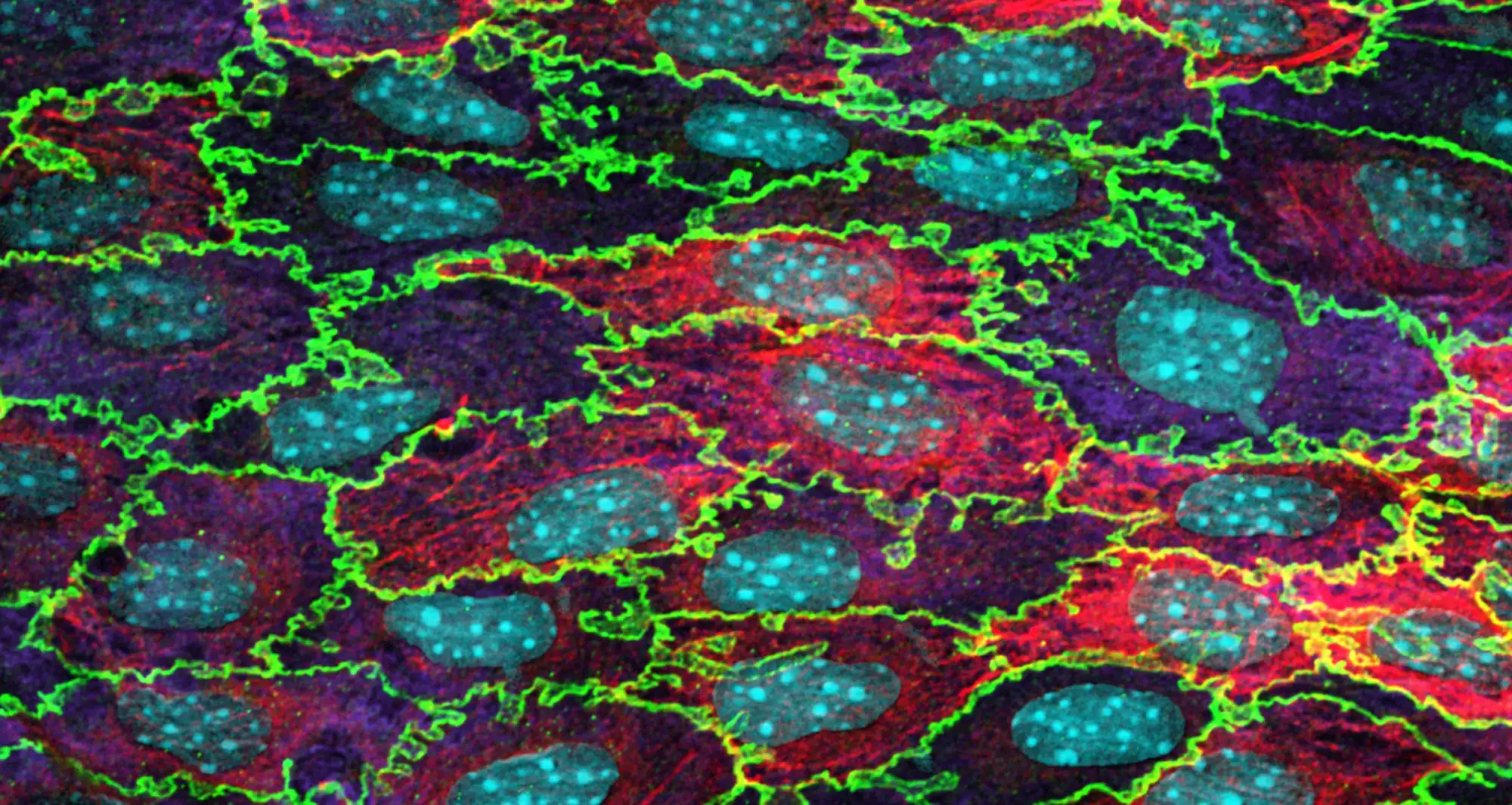
Confocal micrograph of mouse aortic endothelium stained for beta-catenin (green), laminin (purple), smooth muscle actin (red) and Hoechst (cyan). Endothelial cells constitute the inner lining of blood vessels in the heart.
Image courtesy of Florian Alonso, University of Bordeaux/Nikon Small World.

The newly created Paul Walks – MRA Young Investigator Award in Memory of Chad Johnson is part of the alliance’s $9.3 million commitment to melanoma research funding in 2025.
Kelly Kersten, PhD, an assistant professor in the Cancer Metabolism and Microenvironment Program at Sanford Burnham Prebys, was awarded a new type of grant from the Melanoma Research Alliance (MRA). The funding will support Kersten’s research on reactivating “exhausted” immune cells within melanoma tumors to restore their cancer-fighting ability and improve the effectiveness of melanoma immunotherapy.
“Inside tumors, immune cells often lose their strength to attack cancer,” said Kersten. “Our work is focused on understanding and reversing this exhaustion to make therapies more effective for more people.”
The MRA is the world’s leading nonprofit funder of melanoma research. The organization created the Paul Walks – MRA Young Investigator Award in Memory of Chad Johnson to provide support for the next generation of scientists driving innovation against melanoma.
“Our Young Investigator Awards fuel the creativity and drive of early-career scientists whose work can redefine the future of melanoma research.” said Joan Levy, PhD, MRA Chief Science Officer.
The new grant pays tribute to Chad Johnson, a beloved friend and surfer who died from his melanoma diagnosis at age 55. Funding for the award was made possible through “Paul Walks,” a community fundraiser organized by Chad’s lifelong friend, Paul Giobbi.
The Paul Walks – MRA Young Investigator Award in Memory of Chad Johnson is part of MRA’s $9.3 million commitment to fund melanoma research in 2025, supporting more than 30 researchers across the U.S., Europe and Australia. Melanoma remains the deadliest form of skin cancer, with more than 100,000 people expected to be diagnosed this year and one death every hour in the U.S. alone.
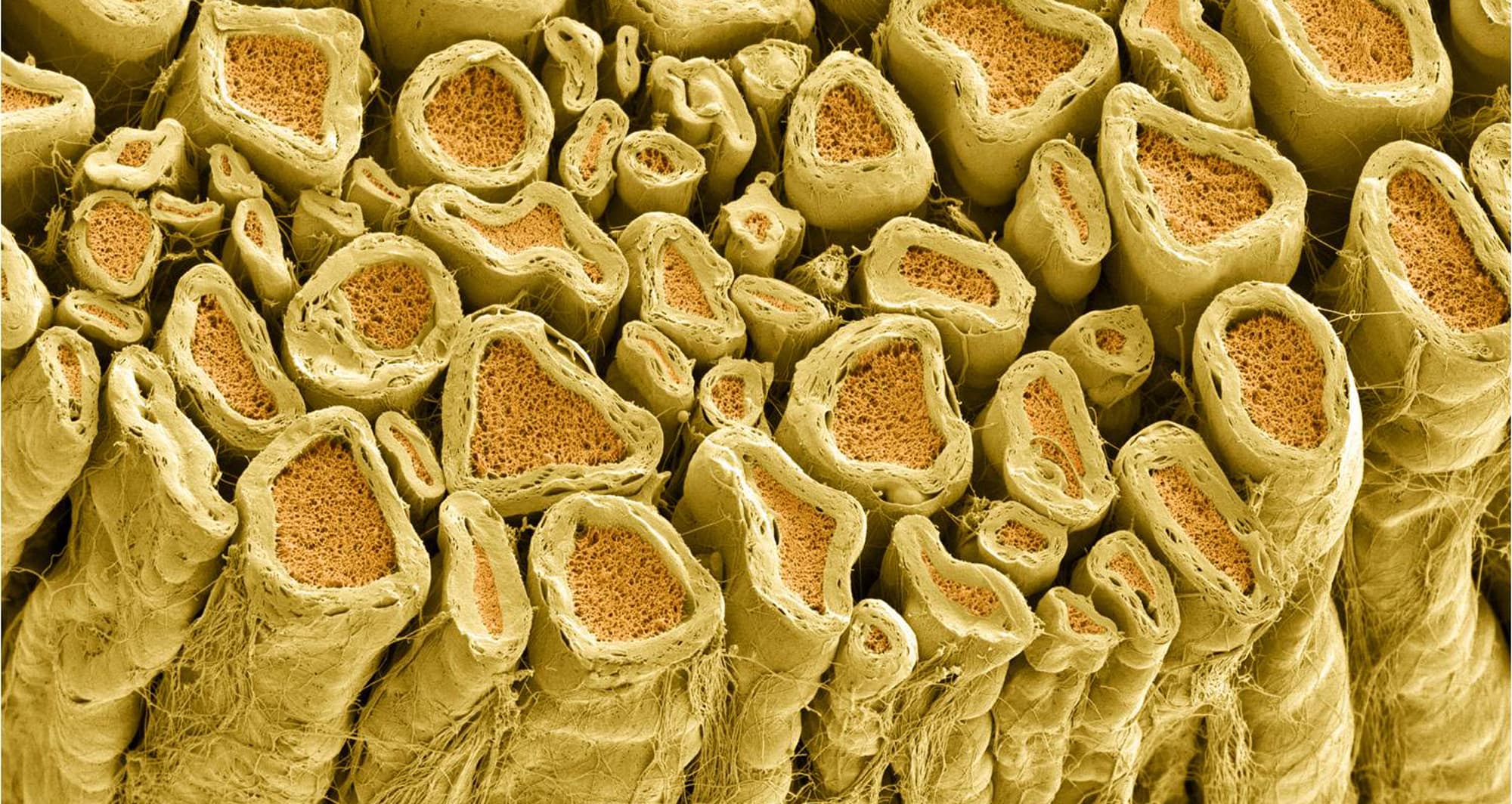
Myelinated axons are depicted in a rat spinal root. Myelin is a type of fat that forms an insulating sheath around the axon to protect it from losing electrical current needed to transmit signals. Axoplasm inside the axon is shown in pink.
Image courtesy of the National Center for Microscopy and Imaging Research at UC San Diego.

We’re on Radiolab! Hudson Freeze, PhD, director of the Sanford Children’s Health Research Center joins the legendary science podcast to recount a discovery that changed biology forever.
For years, scientists thought nothing could live above 73℃/163℉.At that temperature, everything boiled to death. But scientists Tom Brock and Hudson Freeze, PhD, now director of the Sanford Children’s Health Research Center and William W. Ruch Distinguished Chair at Sanford Burnham Prebys, weren’t convinced.
What began as their simple quest to trawl for life in some of the hottest natural springs on Earth would, decades later, change the trajectory of biological science forever, saving millions of lives.
Listen to Freeze recount a transformative moment in his early career on the latest Radiolab podcast.