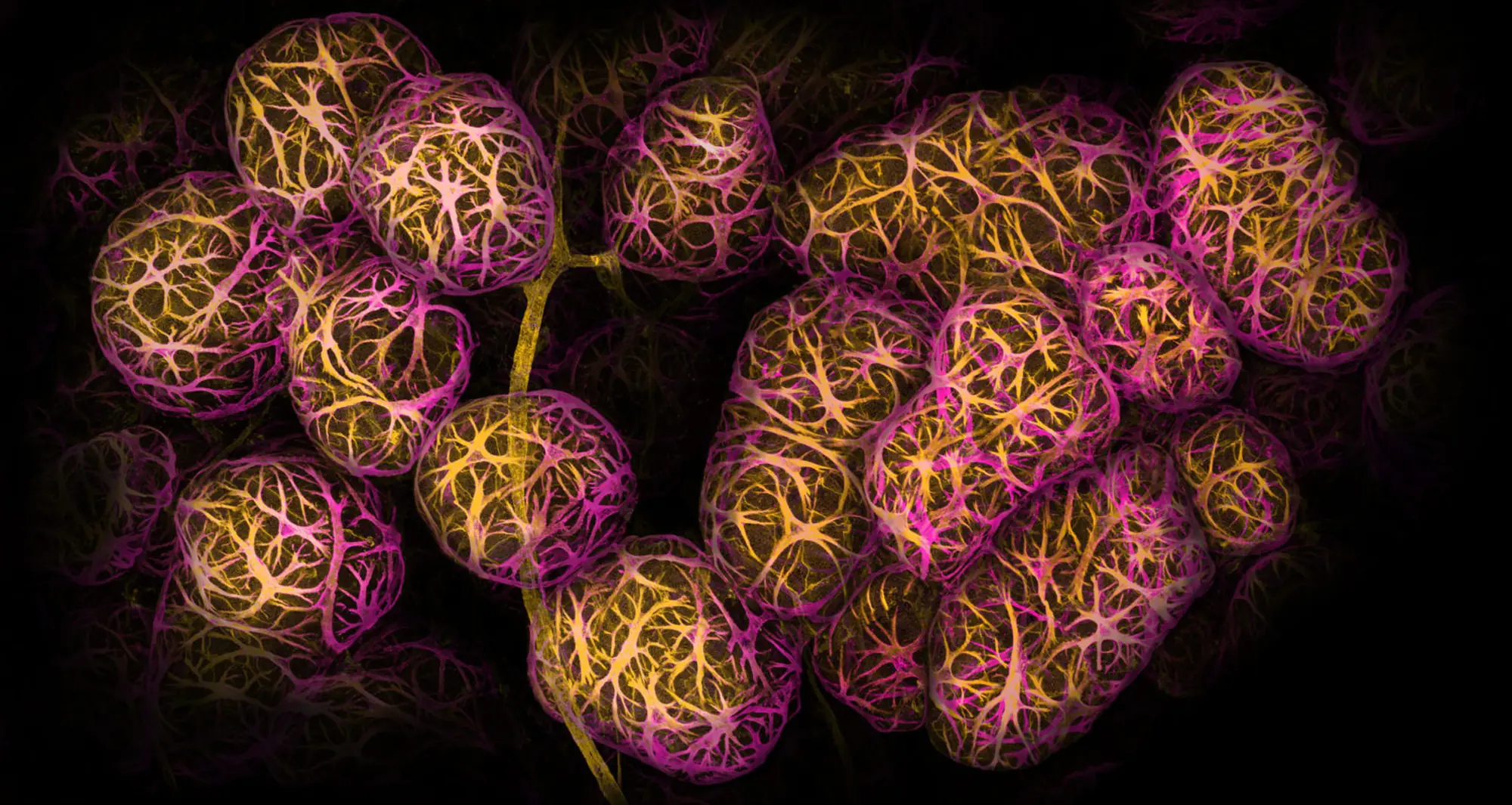
Using confocal microscopy, this image depicts breast tissue showing contractile myoepithelial cells wrapped around milk-producing alveoli.
Image courtesy of Caleb Dawson at Walter and Eliza Hall Institute of Medical Research in Australia and Nikon.
Using confocal microscopy, this image depicts breast tissue showing contractile myoepithelial cells wrapped around milk-producing alveoli.
Image courtesy of Caleb Dawson at Walter and Eliza Hall Institute of Medical Research in Australia and Nikon.
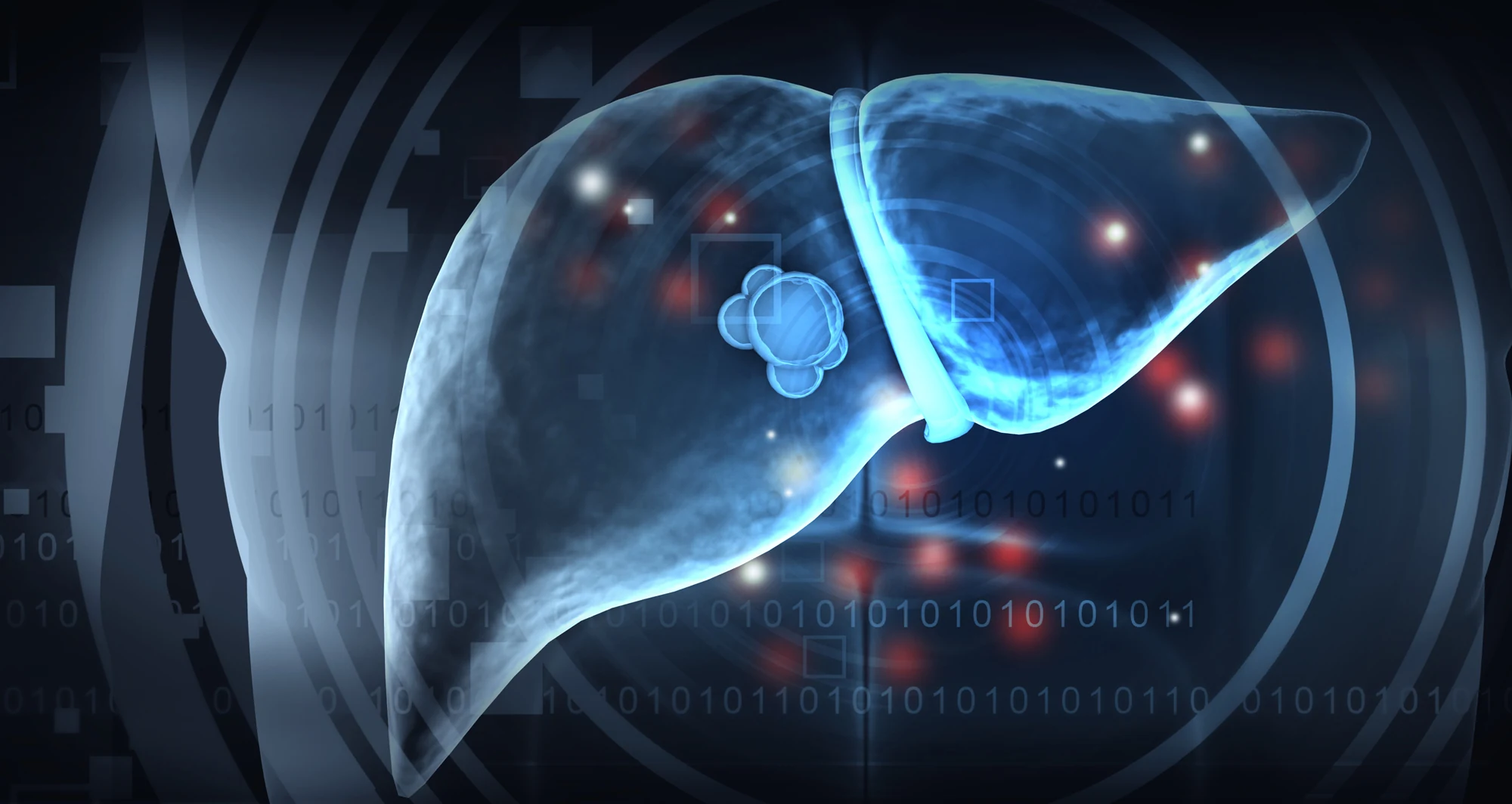
Scientists show how the advanced form of fatty liver disease has monstrous effects on liver cancer risk
Liver cancer has proven to be a tough beast to tame. Experts expected rates of the cancer to decrease following the development of the hepatitis B vaccine in the 1980s, which reduced one of the major risk factors for the disease.
Research in Taiwan showed that its universal infant hepatitis B vaccination program led to young adults experiencing a 35.9% reduction in cases of hepatocellular carcinoma (HCC), the most common liver cancer.
Despite innovation leading to the world’s first cancer-preventing vaccine, incidence of HCC has been on the rise due to a spike in fatty liver disease over recent decades. Lifestyle factors such as high-calorie diets, excessive alcohol consumption and minimal exercise — along with genetic predispositions — can lead to problematic changes in the liver, heart and kidneys.
Specifically in the liver, growing deposits of fat in the tissue can lead over time to an advanced form of fatty liver disease marked by chronic inflammation and the accumulation of thickened scar tissue, a condition known as metabolic-associated steatohepatitis (MASH). MASH significantly increases a patient’s risk of developing HCC.

Debanjan Dhar, PhD, is an associate professor in the Cancer Genome and Epigenetics Program.
In a paper published January 1, 2025, in Nature, scientists at Sanford Burnham Prebys, the University of California San Diego, Curtin University, the University of Pennsylvania and The Liver Cancer Collaborative, demonstrated that MASH damages the DNA of liver cells. The study also linked these changes to the development of liver cancer.

Peter Adams, PhD, is the director of the Cancer Genome and Epigenetics Program.
“DNA damage from MASH causes liver cells to stop dividing and enter a zombie-like state called senescence,” said Debanjan Dhar, PhD, associate professor in the Cancer Genome and Epigenetics Program at Sanford Burnham Prebys and coauthor on the study. “This study’s results demonstrate that some of these cells later exit senescence and are likely to become cancerous due to their accumulation of damage and mutations.”
“In the future, we can apply what we’ve learned to study potential opportunities to prevent or repair DNA damage from MASH to reduce patients’ risk of developing liver cancer,” said Peter Adams, PhD, director of the Cancer Genome and Epigenetics Program at Sanford Burnham Prebys and coauthor on the study.
Michael Karin, PhD, Distinguished Professor in the Department of Pharmacology at the University of California San Diego School of Medicine, is the senior and corresponding author on the study.
Li Gu, PhD, a former postdoctoral fellow in the Karin lab, shares first authorship of the study with visiting scientist Yahui Zhu.
Additional authors include:
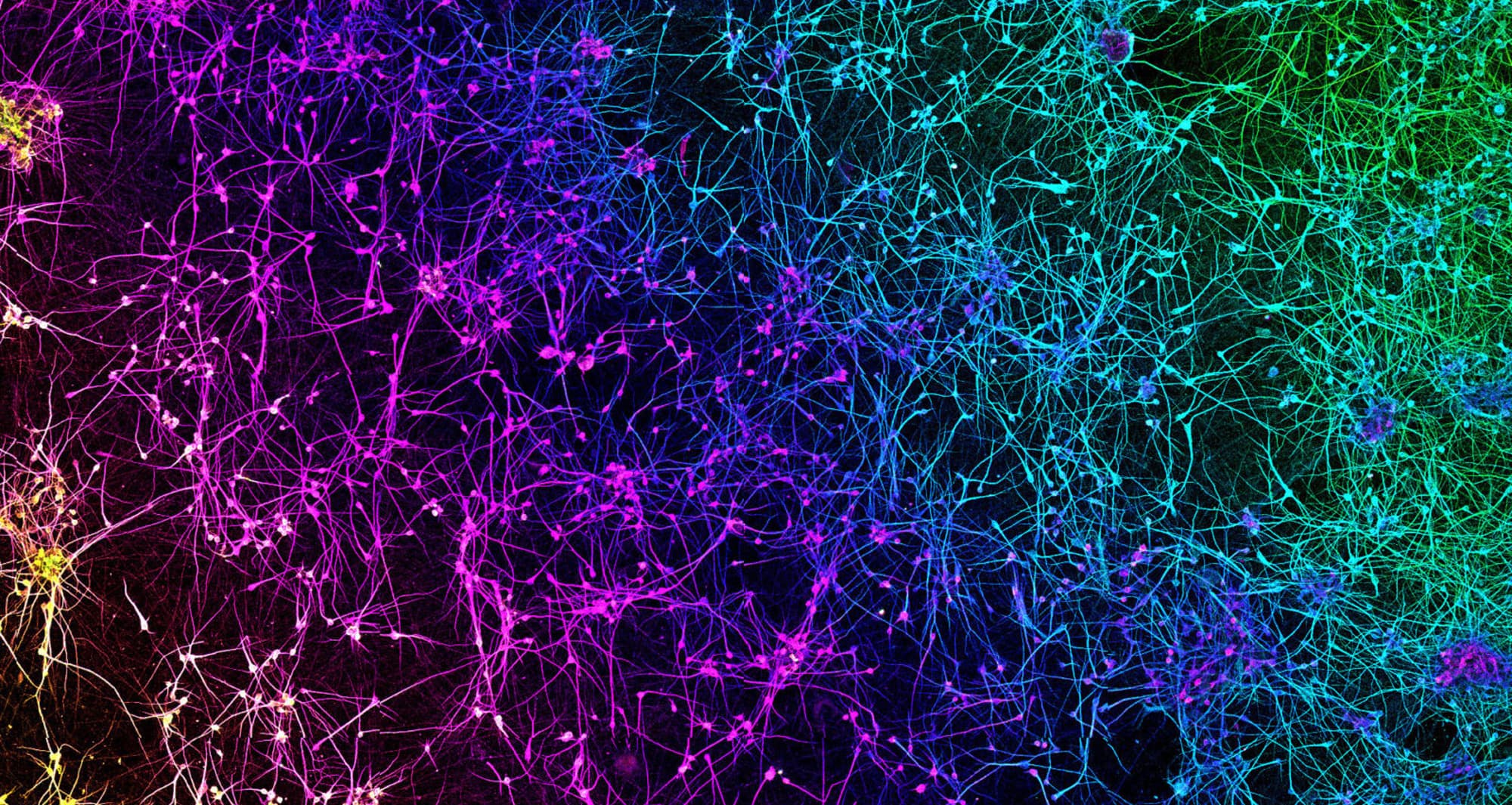
A network of dopaminergic neurons generated from human stem cells. Dopaminergic neurons regulate many brain functions, including voluntary movement, learning and reward and working memory.
Image courtesy of Nick Gatford, University of Oxford and Nikon Small World.
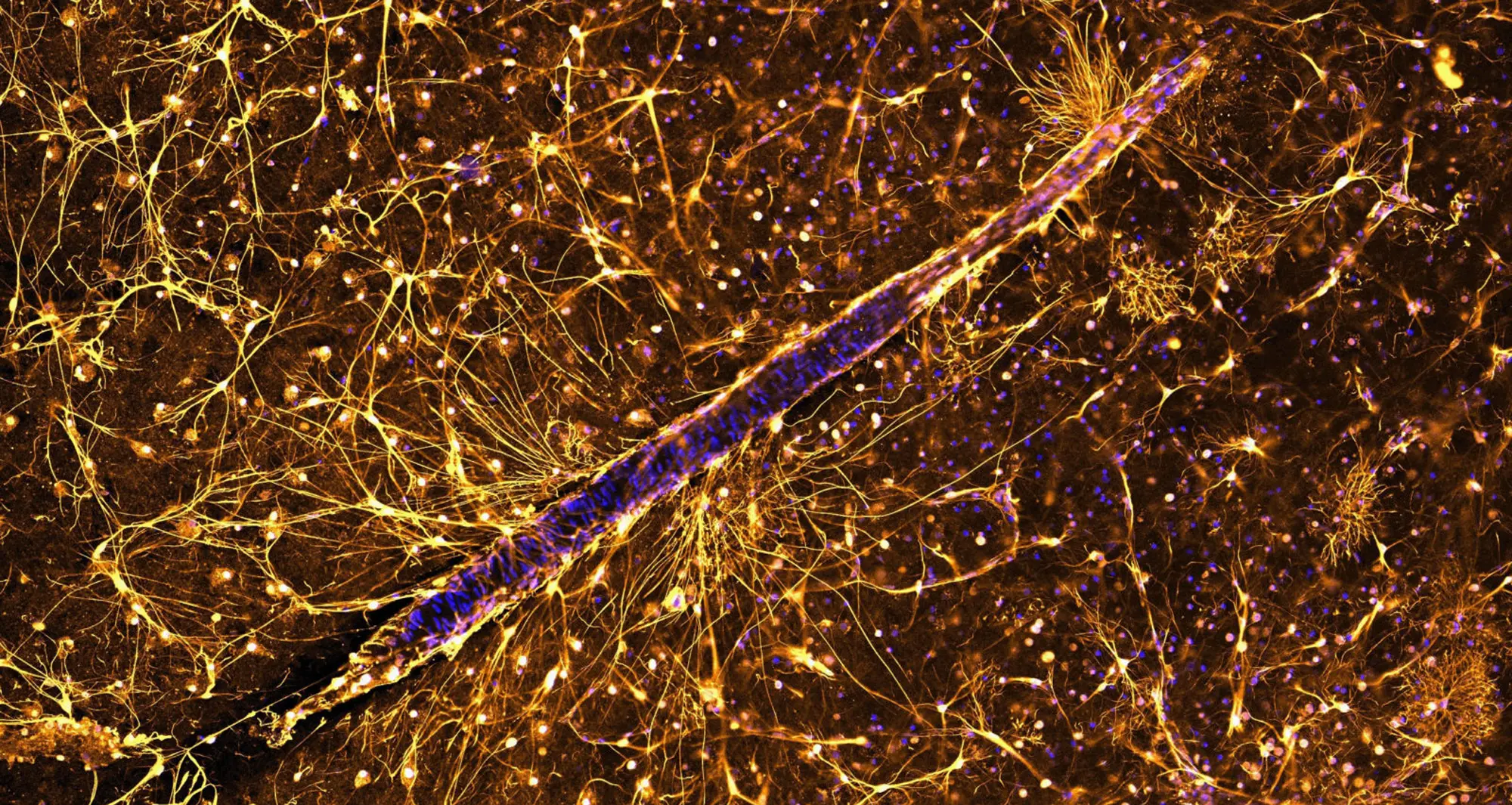
Astrocytes surrounding a blood vessel in a thin slice of human brain.
Image courtesy of Anja de Lange, University of Cape Town and Nikon Small World.
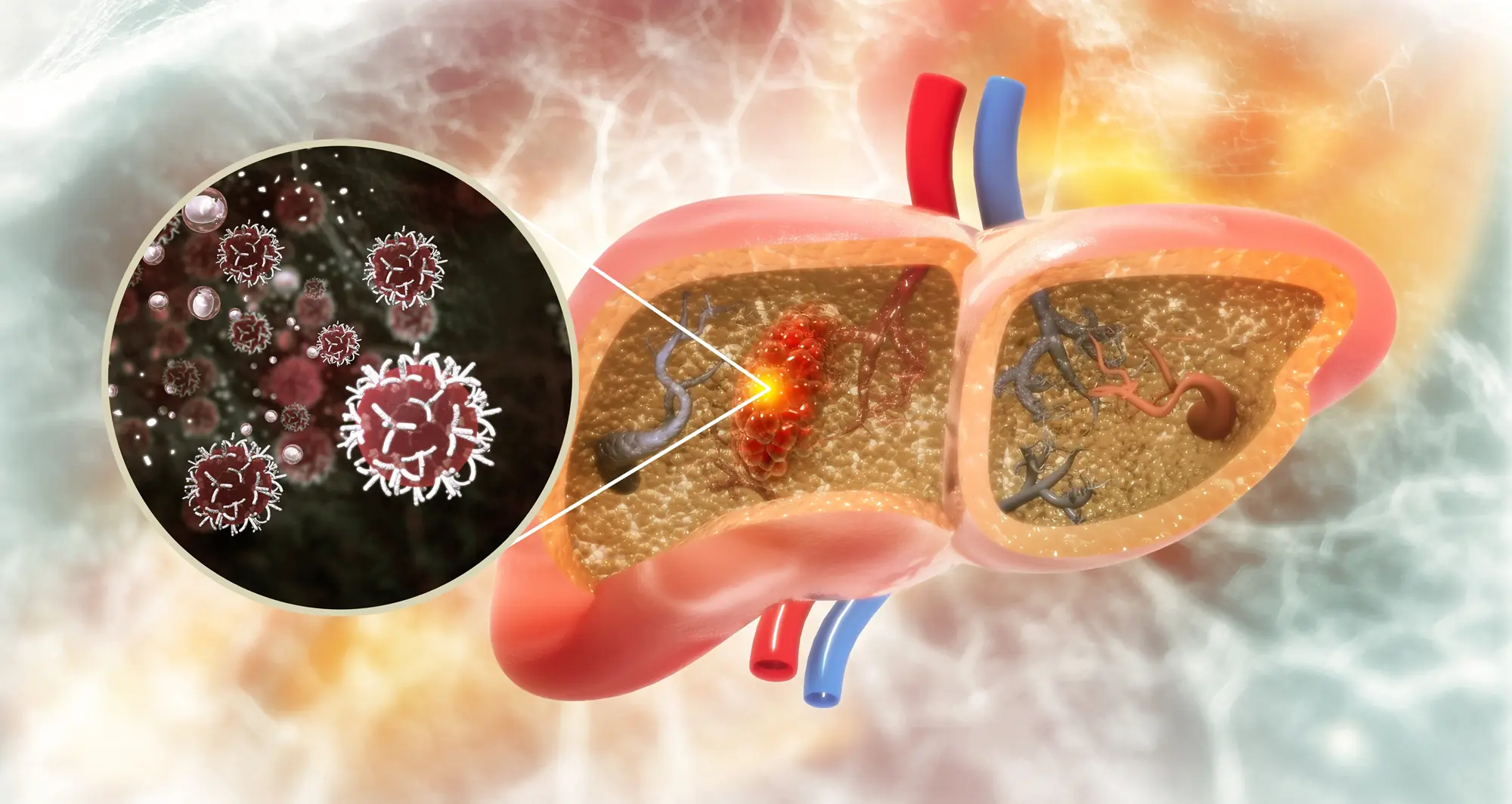
Understanding the crucial ingredient in bile may unlock the potential of treatments that help patients’ immune systems eliminate cancer
Hepatocellular carcinoma (HCC) is the most common liver cancer and a growing threat to public health across the globe due to the rising rate of fatty liver disease.
Liver cancer is difficult to treat as it often causes few if any symptoms early on, so it tends to be diagnosed at later, more aggressive stages. While immunotherapies that supercharge patients’ immune systems have proven effective in some cancers, this approach has had limited success in patients suffering from HCC or other forms of the disease.
Scientists are investigating the unique qualities of different tissues that may explain why the effectiveness of immunotherapy varies depending on the location of a tumor. The liver is known to have a flexible immune system capable of defending itself when necessary while not overreacting to a constant flood of foreign materials from digesting food, including metabolic byproducts from bacteria residing in the gut microbiome.
Transplant surgeons see the unique properties of the liver’s immune system firsthand when transplanted livers are typically integrated by recipients with only a low dose of immunosuppressive drugs. This ability to maintain immune tolerance, however, may reduce the ability of the liver’s immune system to find and destroy cancer cells, even when that capability is enhanced by immunotherapy.
In a paper published January 9, 2025, in Science, scientists at Sanford Burnham Prebys, the Salk Institute, the University of California San Diego, Columbia University Irving Medical Center, Memorial Sloan Kettering Cancer Center and the Geisel School of Medicine at Dartmouth, found that a critical ingredient in bile hinders the liver’s immune response against cancer.
Bile is a fluid made by the liver that assists in breaking down fats during digestion. This function is made possible by steroidal acids known as bile acids. The scientists found an increased amount of bile acids in tumor samples from patients with HCC. The team also found that genes involved in creating bile acids were being transcribed to make proteins and enzymes at an abnormally high rate in human samples and in mice genetically modified to develop liver cancer.
The authors went on to remove genes related to bile acid construction to demonstrate that mice without these blueprints developed fewer, smaller tumors. In addition, the liver’s T cells — the primary anti-tumor immune cells — were able to dig deeper into tumors and persist for longer without the immunosuppressive effects of certain bile acids.
“These findings underscore a new appreciation for the influence of bile acids on the liver’s immune system,” said Debanjan Dhar, PhD, associate professor in the Cancer Genome and Epigenetics Program at Sanford Burnham Prebys and coauthor on the study. More research is needed to test the potential use of drugs to directly inhibit certain bile acids or bile acid receptors as a therapeutic strategy to reduce liver cancer growth.

Debanjan Dhar, PhD, is an associate professor in the Cancer Genome and Epigenetics Program at Sanford Burnham Prebys.

Peter Adams, PhD, is the director of the Cancer Genome and Epigenetics Program at Sanford Burnham Prebys.
It may also be possible to achieve this effect through dietary changes that alter the microbiome and result in modified bile acid production. Based on their findings, the research team suggests that this could be done by using ursodeoxycholic acid, a bile acid that currently is used to treat an autoimmune condition called primary biliary cholangitis. The acid is found at high levels in bear bile, which has served for thousands of years as a treatment in traditional Chinese medicine.
“Given the safety profile of ursodeoxycholic acid and the limited effectiveness of immunotherapy on liver cancer, this study shows significant potential for testing this bile acid as a combination treatment for patients with HCC,” said Peter Adams, PhD, director of the Cancer Genome and Epigenetics Program at Sanford Burnham Prebys and coauthor on the study.
Susan Kaech, PhD, NOMIS Chair, professor and director of the NOMIS Center for Immunobiology and Microbial Pathogenesis at the Salk Institute is the senior and corresponding author on the study.
Siva Karthik Varanasi, PhD, assistant professor at the UMass Chan Medical School and a former postdoctoral fellow in the Kaech lab at the Salk Institute, is first author on the manuscript.
Additional authors include:
Wolfram Goessling, MD, PhD, the Robert H. Ebert Associate Professor of Medicine and associate professor of Health Sciences and Technology at Harvard Medical School, authored a Perspective article on the new study in Science called, “Ena-bile-ing liver cancer growth.”
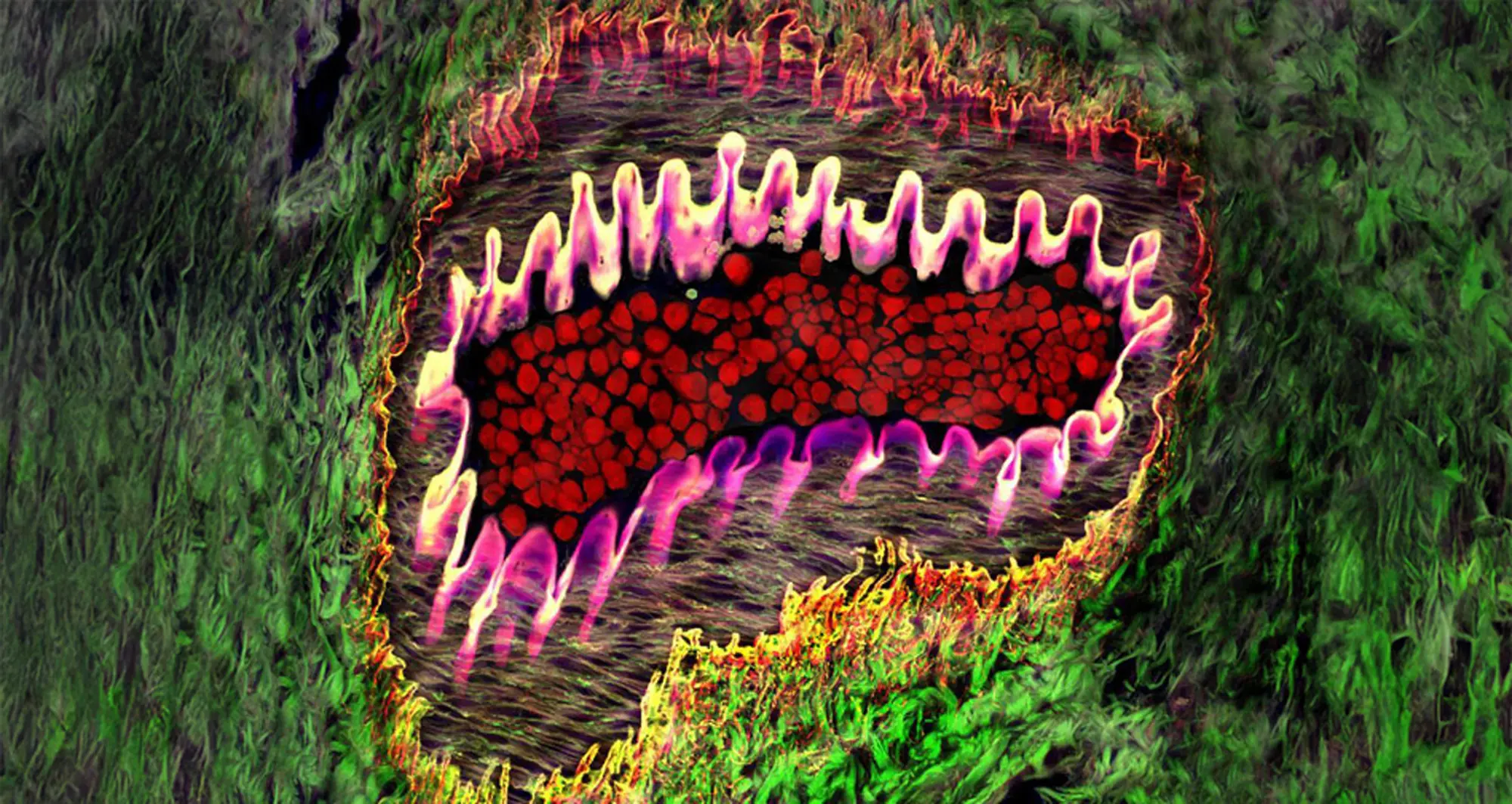
Healthy arteriole in eye, with tough, flexible elastin wall (pink), red blood cells (red) and supporting collagen fibers (web-like netting) surrounded by yellow and green areas.
Image courtesy of Donald Pottle, The Schepens Eye Research Institute, Boston and Bioscapes.

Hypophosphatasia (HPP) is a rare genetic disorder in which bones and teeth fail to take up sufficient calcium and phosphorus needed to achieve proper hardness and strength. Defective mineralization results in bones that are soft and prone to fracture and deformity, and the loss of teeth. Occasionally, HPP can cause death due to complications.
Prevalence varies by severity and age of onset. It is rarest but most severe at birth (1 in 100,000 live births), with lower prevalence and milder forms in later years. The condition can manifest at any age.
The cause of HPP is a mutation in an enzyme called tissue-nonspecific alkaline phosphatase (TNAP), which plays a critical role in skeletal and dental mineralization. In 2015, an enzyme replacement therapy developed by José Luis Millán, PhD, a professor in the Human Genetics Program at Sanford Burnham Prebys, was approved to treat pediatric onset HPP, dramatically improving patients’ lifespan and quality of life.
But the effects of TNAP deficiency appears to extend beyond faulty mineralization. HPP patients also experience altered immune responses, suggesting TNAP might have a role in immune cells.
Recently, Soft Bones, an advocacy group for HPP patients, awarded Valeria Guglielmi, PhD, a postdoctoral associate in Maximiliano D’Angelo’s lab, with a one-year, $25,000 seed grant to further investigate the involvement of TNAP in inflammatory responses and immune cell functions.
“This study is in line with my broad interest for immune cells and their contribution to tissue homeostasis and diseases,” said Guglielmi. “I am excited to explore an entirely new area of investigation on HPP.
“Indeed, very little is known about the role of TNAP in the immune system and only a few studies have provided evidence of TNAP involvement in immune cell function. By uncovering how TNAP deficiency affects inflammatory responses, our research represents the first step toward designing interventions to improve immune system dysfunctions in HPP patients.”
Read Soft Bones’ full news release on the award to Guglielmi on Facebook and Instagram.

A bacteria linked to longevity was found to feast on lactate only when the meal contains the metallic side dish
In a new paper published December 30, 2024, in PNAS, study coauthor Dmitry A. Rodionov, PhD, research assistant professor in the Immunity and Pathogenesis Program at Sanford Burnham Prebys, and colleagues, studied how Eubacterium limosum contribute to a healthy human gastrointestinal microbiome by metabolizing lactate.
Lactate or lactic acid is a normal byproduct that is created as our cells generate energy. Lactate can be found in the guts of healthy adults at low concentrations because microbes such as E. limosum make a meal of much of it, preventing the abnormal accumulation sometimes found in patients suffering from ulcerative colitis and other gut-related disorders.
Rodionov and his colleagues examined how E. limosum bacteria break down lactate into short-chain fatty acids (SCFAs) and were surprised to find that the metabolic process depends on two tungsten-containing enzymes.
The authors suggest that their findings are a tipoff that tungsten might be an overlooked micronutrient in the human gut microbiome and may contribute in unappreciated ways to overall human health.

New research on four variants in the EPHA1 gene reveals how its genetic typos may contribute to risk of dementia
Upon inspecting the DNA sequences in patients suffering from Alzheimer’s disease, scientists have found evidence of an inconspicuous conspirator.
The EPHA1 gene contains the blueprint for the EPHA1 receptor protein, one of 14 such receptor proteins in the Eph receptor family. Relatively little is known about EPHA1 when compared to many of its siblings, making it difficult for researchers to ascertain why changes in its source code would contribute to such a debilitating disease.
Scientists at Sanford Burnham Prebys published results on December 18, 2024, in the Journal of Biological Chemistry, detailing the effects of four miniature mutations of just a single typo each in the sequence of nucleotides forming the EPHA1 gene.
These seemingly minor mutations are known as single nucleotide polymorphisms (SNPs), and they can lead to larger issues depending on where the typos fall in the sequence of a gene. The Sanford Burnham Prebys team focused on four missense mutations that are caused when SNPs result in different amino acids being used to build the EPHA1 receptor protein.
“Our data show that all four Alzheimer’s mutations we have characterized disrupt EPHA1 physiological signaling, and that the specific effects depend on the particular mutation,” said Elena Pasquale, PhD, professor in the Cancer Metabolism and Microenvironment Program at Sanford Burnham Prebys.
The team reported that the functional consequences of EPHA1 missense mutations identified in patients suffering from Alzheimer’s disease included misplacement of EPHA1 within cells, decreased protein stability and dysregulated signaling.
“To continue advancing knowledge on this topic, more work is needed to uncover the physiological role of the different EPHA1 signaling features and how their disruption may lead to neurodegeneration,” said Pasquale.
Additional authors on the study from Sanford Burnham Prebys include Mike Matsumoto and Sara Lombardi, PhD. Maricel Gomez-Soler, PhD, now works at Crinetics Pharmaceuticals in San Diego. Bernhard C. Lechtenberg, PhD, now works at the Walter and Eliza Hall Institute of Medical Research in Parkville, Australia.
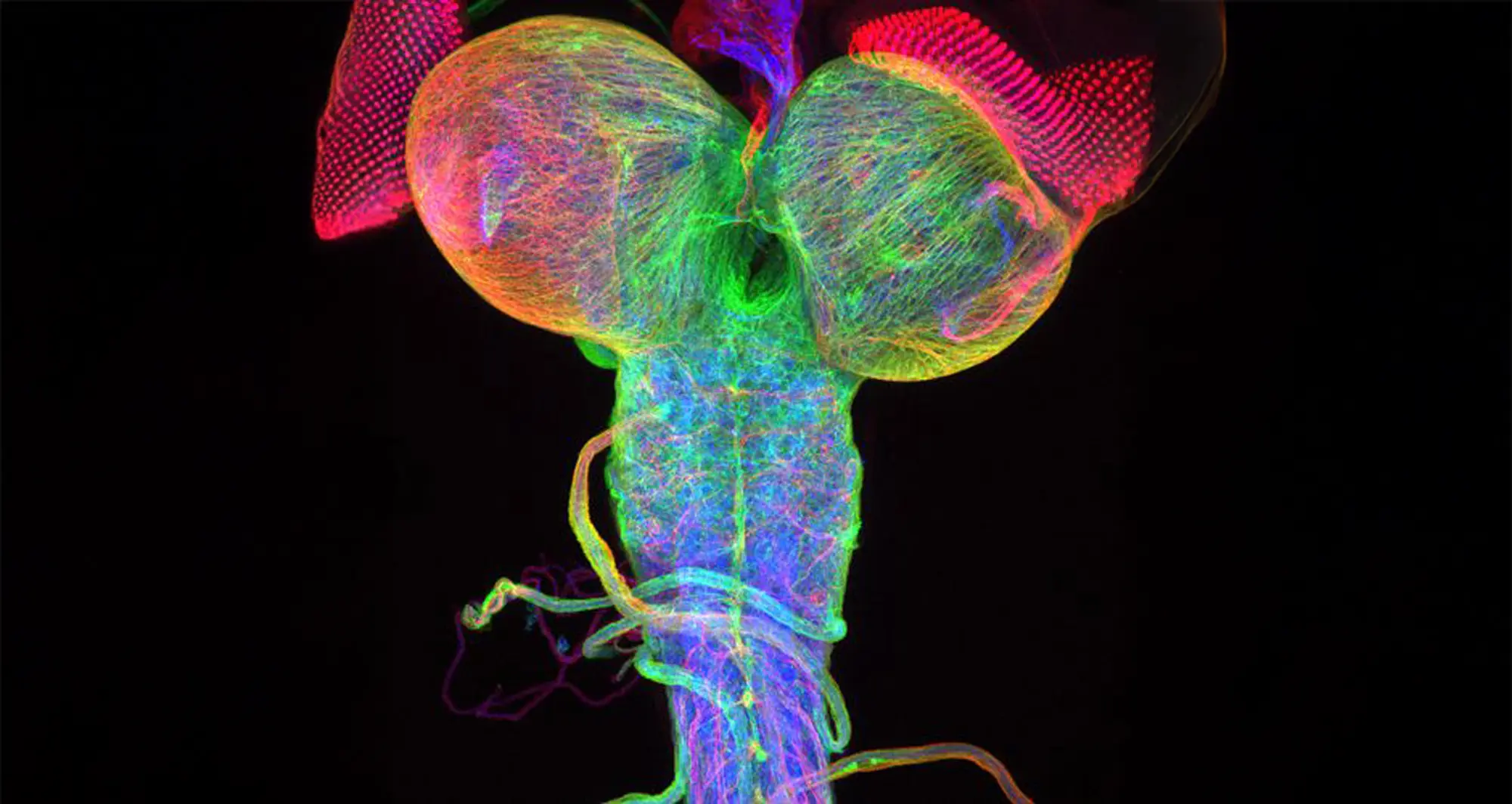
Beta-tubulin expression of a Drosophila (fruit fly) third instar larval brain, with attached eye imaginal discs.
Image courtesy of Christian Klambt and Imke Schmidt, University of Munster, Germany and Bioscapes.