Research
People
Research Team at Sanford Burnham Prebys
Ronnie Borland PhD
- Postdoctoral Associate
Shrawan Chavan PhD
- Postdoctoral Associate
Don Clarke PhD
- Medical Research Scientist
Robert Fraumeni BSc
- Research Associate II
James Paulson PhD
- Investigator
Melina Melameka Pesi Safi BSc
- Research Associate
Mayank Saraswat PhD
- Senior Staff Scientist
Affiliates
Jeffrey Esko PhD
- Distinguished Professor
Department of Cellular & Molecular Medicine
UC San Diego
Jeffrey Fried MD
- Acute Care Physician
Cottage Hospital Santa Barbara
Douglas Heithoff PhD
- Project Scientist
UC Santa Barbara
Dzung Le MD, PhD
- Clinical Professor, Pathology
Director, Coagulation Laboratory
Associate Director
Clinical Hematology Laboratory
Associate Director
Blood Bank and Transfusion Medicine
UC San Diego
Michael J. Mahan PhD
- Professor
Molecular, Cellular, and Developmental Biology
UC Santa Barbara
Victor Nizet MD
- Professor & Vice Chair for Basic Research
Department of Pediatrics
UC San Diego
Chief, Division of Host-Microbe Systems & Therapeutics
Professor, Skaggs School of Pharmacy & Pharmaceutical Sciences
Gabriel Wardi MD
- Assistant Professor
Emergency Medicine
UC San Diego
News
Employment
Interested, qualified applicants for Research Technicians, Postdoctoral Associates, and Medical Research positions should email their résumé or Curriculum Vitae including names and contacts for up to three references to Araceli Ambert.
Contact
The Marth Laboratory
Sanford Burnham Prebys
10901 N. Torrey Pines Rd.
La Jolla, CA 92037
Building 5
Laboratory Rooms: 5221, 5215, 5254, 5256
Office Rooms: 5248, 5250
Laboratory Phone Number: (858) 795-5109
Office Phone Number: (858) 795-5110
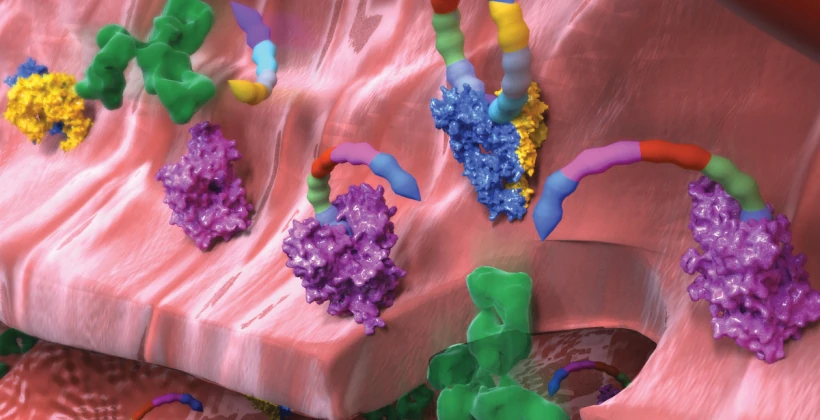 In autoimmune disease, the immune system attacks various parts of the body. This can lead to organ and systems failure, and possibly death. The reasons for this are mostly unknown. This laboratory is investigating the molecular origins of autoimmune disease in part by identifying those self-targets of the immune system. This includes metabolic and genetic factors resulting in cell surface changes often including aberrant glycan linkages produced among somatic and non-hematopoietic cell types. Previously we discovered an unexpected autoimmune disease mechanism with tissue destruction that although it is diagnosed as autoimmune disease, the pathology is independent of adaptive immune function. Our ongoing research includes studies of human patients and mouse models in collaboration with hospitals and other medical research institutes.
In autoimmune disease, the immune system attacks various parts of the body. This can lead to organ and systems failure, and possibly death. The reasons for this are mostly unknown. This laboratory is investigating the molecular origins of autoimmune disease in part by identifying those self-targets of the immune system. This includes metabolic and genetic factors resulting in cell surface changes often including aberrant glycan linkages produced among somatic and non-hematopoietic cell types. Previously we discovered an unexpected autoimmune disease mechanism with tissue destruction that although it is diagnosed as autoimmune disease, the pathology is independent of adaptive immune function. Our ongoing research includes studies of human patients and mouse models in collaboration with hospitals and other medical research institutes.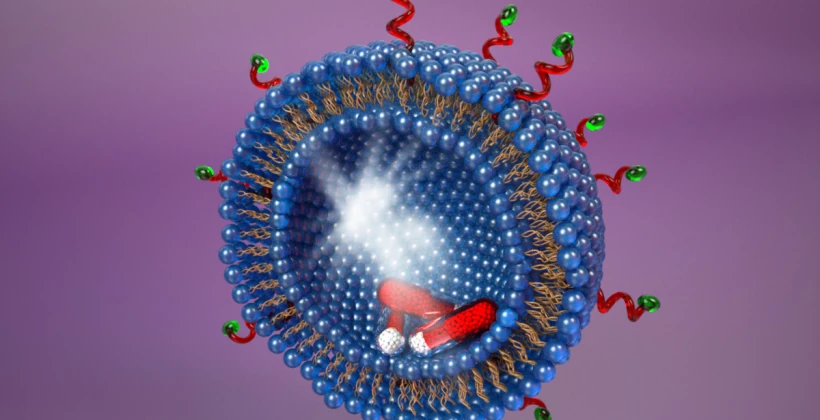 Cancer is among the most deadly of diseases. In cancer, cells become immortal and grow in an unrestricted manner, resulting in tumors, metastasis, sickness, and often, premature death. The body’s immune system is capable of detecting cancer, however it is often functionally disabled by mechanisms including what is now known as checkpoint inhibition. With the advent of approaches to reduce or eliminate checkpoint inhibition mechanisms, along with various ways to reprogram the immune system in cancer, it is now possible to investigate and the human immune response to cancer. Our approach is to use the cancer-associated changes to the cell surface to bait for binding and isolation human antibodies to multiple different types of cancer for further development with the goal of isolating and using components of the human immune system, including anti-tumor antibodies, to achieve significant diagnostic and therapeutic advances.
Cancer is among the most deadly of diseases. In cancer, cells become immortal and grow in an unrestricted manner, resulting in tumors, metastasis, sickness, and often, premature death. The body’s immune system is capable of detecting cancer, however it is often functionally disabled by mechanisms including what is now known as checkpoint inhibition. With the advent of approaches to reduce or eliminate checkpoint inhibition mechanisms, along with various ways to reprogram the immune system in cancer, it is now possible to investigate and the human immune response to cancer. Our approach is to use the cancer-associated changes to the cell surface to bait for binding and isolation human antibodies to multiple different types of cancer for further development with the goal of isolating and using components of the human immune system, including anti-tumor antibodies, to achieve significant diagnostic and therapeutic advances.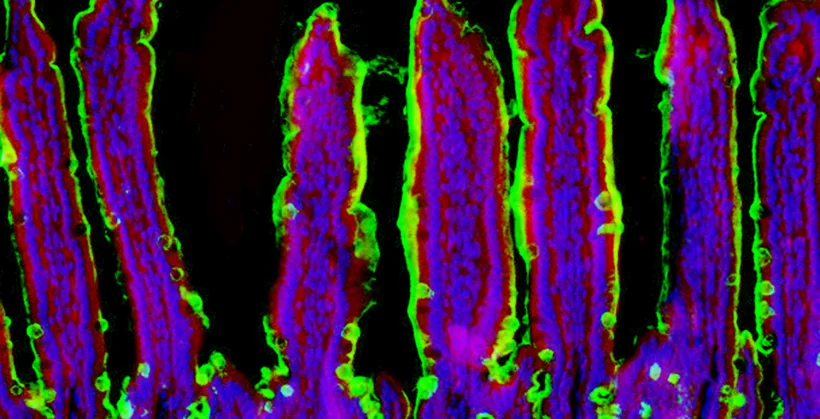 Intestinal inflammation is the basis of colitis. The incidence and prevalence of colitis and the inflammatory bowel diseases (IBDs) are increasing, yet the origins of these life-threatening syndromes predominantly include environmental factors as deduced from studies of genetic twins and other human populations. Possible causal links include repeated exposure to microbial pathogens. Foodborne infections represent a common and escalating human health problem that are likely involved in the early origins of colitis and the IBDs. To investigate further, we have developed and characterized the first animal model of environmentally-induced colitis. This was achieved by modeling recurrent human food poisoning using low-dose nonlethal gastrointestinal infections of Salmonella, a major food-borne pathogen in the human population. We discovered that repeated exposure over the lifetime induced progressive inflammation of intestinal tissues, especially the colon. Although the host effectively cleared the pathogen weeks before reinfection, inflammation and intestinal tissue damage increased in severity with additional infections. We have further identified pathogenic mechanisms to include the host response to infection resulting in reduction of the anti-inflammatory enzyme alkaline phosphatase, as well as the induction of proteolytic remodeling resulting in the degradation of the protective colonic epithelial mucosal barrier. Augmenting alkaline phosphatase and inhibiting serine protease activity are both capable of reducing the onset and severity of colitis. Both are achieved using available drugs currently used for other purposes and which may upon repurposing represent effective approaches to human colitis and the IBDs.
Intestinal inflammation is the basis of colitis. The incidence and prevalence of colitis and the inflammatory bowel diseases (IBDs) are increasing, yet the origins of these life-threatening syndromes predominantly include environmental factors as deduced from studies of genetic twins and other human populations. Possible causal links include repeated exposure to microbial pathogens. Foodborne infections represent a common and escalating human health problem that are likely involved in the early origins of colitis and the IBDs. To investigate further, we have developed and characterized the first animal model of environmentally-induced colitis. This was achieved by modeling recurrent human food poisoning using low-dose nonlethal gastrointestinal infections of Salmonella, a major food-borne pathogen in the human population. We discovered that repeated exposure over the lifetime induced progressive inflammation of intestinal tissues, especially the colon. Although the host effectively cleared the pathogen weeks before reinfection, inflammation and intestinal tissue damage increased in severity with additional infections. We have further identified pathogenic mechanisms to include the host response to infection resulting in reduction of the anti-inflammatory enzyme alkaline phosphatase, as well as the induction of proteolytic remodeling resulting in the degradation of the protective colonic epithelial mucosal barrier. Augmenting alkaline phosphatase and inhibiting serine protease activity are both capable of reducing the onset and severity of colitis. Both are achieved using available drugs currently used for other purposes and which may upon repurposing represent effective approaches to human colitis and the IBDs. The origins of Type 1 and Type 2 diabetes are also mysterious. As with other common diseases, population studies indicate a minor role for genetic variation in the onset of Type 2 diabetes– again indicating that the onset of these diseases is not predominantly determined by your genes. We discovered that the molecular origins of obesity-associated Type 2 diabetes emanate from pancreatic beta cell dysfunction caused by nutritional and metabolic alterations in the presence of high levels of circulating lipids (fats). Disruption of pancreatic beta cell glucose transport in individuals due to excessive lipid levels was linked to the onset of (obesity-associated) Type 2 diabetes, with the elimination of glucose-stimulated insulin secretion and impaired insulin action together disrupting glucose sensing and cellular glycolysis. This discovery helps to explain why ‘diabetes genes’ are not likely to play a significant role and why investigating ‘diabetes resistance’ genes’ could be a more effective approach to develop treatments to reverse this potentially fatal condition. Loss of beta cell function is also the basis of Type 1 diabetes (an autoimmune disease). In Type 1 diabetes, the body’s own immune system attacks its pancreatic beta cells and destroys them, thereby leading to insulin deficiency. We are currently investigating the reason for this and have acquired unexpected data implicating changes at the beta cell surface that render these cells more likely to undergo immunological attack. We are testing this hypothesis by altering the molecular structures existing on the beta cell surface to determine if such changes can provide protection of beta cells from immune-based destruction and the onset of diabetes.
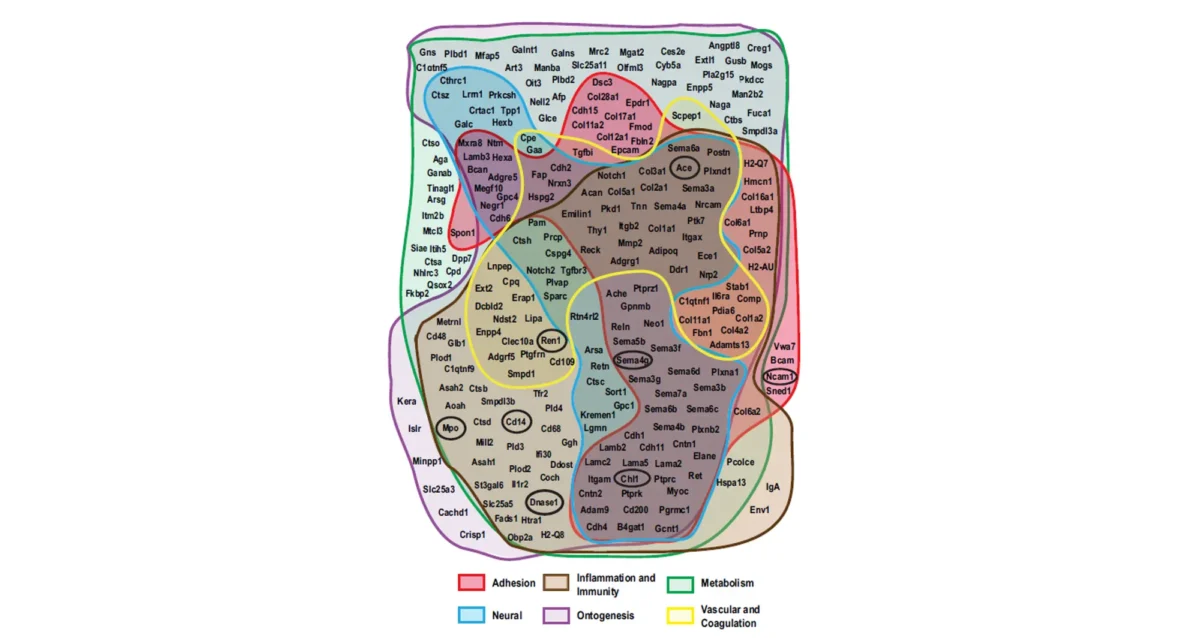
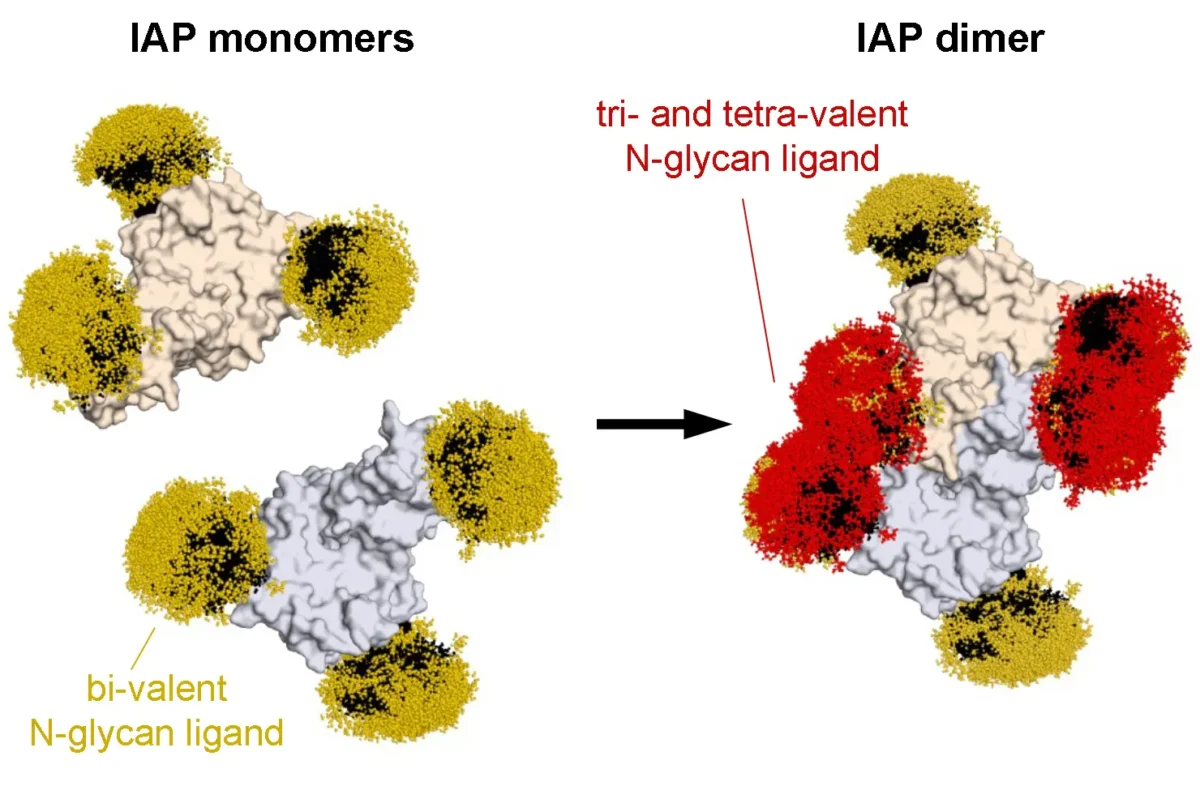
The origins of Type 1 and Type 2 diabetes are also mysterious. As with other common diseases, population studies indicate a minor role for genetic variation in the onset of Type 2 diabetes– again indicating that the onset of these diseases is not predominantly determined by your genes. We discovered that the molecular origins of obesity-associated Type 2 diabetes emanate from pancreatic beta cell dysfunction caused by nutritional and metabolic alterations in the presence of high levels of circulating lipids (fats). Disruption of pancreatic beta cell glucose transport in individuals due to excessive lipid levels was linked to the onset of (obesity-associated) Type 2 diabetes, with the elimination of glucose-stimulated insulin secretion and impaired insulin action together disrupting glucose sensing and cellular glycolysis. This discovery helps to explain why ‘diabetes genes’ are not likely to play a significant role and why investigating ‘diabetes resistance’ genes’ could be a more effective approach to develop treatments to reverse this potentially fatal condition. Loss of beta cell function is also the basis of Type 1 diabetes (an autoimmune disease). In Type 1 diabetes, the body’s own immune system attacks its pancreatic beta cells and destroys them, thereby leading to insulin deficiency. We are currently investigating the reason for this and have acquired unexpected data implicating changes at the beta cell surface that render these cells more likely to undergo immunological attack. We are testing this hypothesis by altering the molecular structures existing on the beta cell surface to determine if such changes can provide protection of beta cells from immune-based destruction and the onset of diabetes. Sepsis is a common and deadly syndrome linked to an infection of the bloodstream. Millions of people are diagnosed with sepsis each year, and on average 30% will die or become disabled from the severe inflammation and coagulation abnormalities that result. Sepsis is now more prevalent worldwide than cancer. We have been studying a receptor system at the nexus of disease pathogenesis in the body that provides in some cases host protection during sepsis, while in other cases it plays a detrimental role. This receptor system controls inflammatory responses, blood coagulation and thrombosis, all key processes contributing to the pathology of sepsis. We have further learned how to manipulate this receptor system to reduce inflammation and thrombosis to increase survival in both Gram-positive and Gram-negative sepsis. These findings may support the development of the first new effective drugs to treat sepsis in over three decades. These discoveries focus on the rate of protein aging and turnover in the blood, and further identify specific enzymes and small molecule drugs already available that may be repurposed for targeted therapies of sepsis. We continue to work to establish this potential in collaboration with our colleagues at multiple hospitals and other research institutes.
Sepsis is a common and deadly syndrome linked to an infection of the bloodstream. Millions of people are diagnosed with sepsis each year, and on average 30% will die or become disabled from the severe inflammation and coagulation abnormalities that result. Sepsis is now more prevalent worldwide than cancer. We have been studying a receptor system at the nexus of disease pathogenesis in the body that provides in some cases host protection during sepsis, while in other cases it plays a detrimental role. This receptor system controls inflammatory responses, blood coagulation and thrombosis, all key processes contributing to the pathology of sepsis. We have further learned how to manipulate this receptor system to reduce inflammation and thrombosis to increase survival in both Gram-positive and Gram-negative sepsis. These findings may support the development of the first new effective drugs to treat sepsis in over three decades. These discoveries focus on the rate of protein aging and turnover in the blood, and further identify specific enzymes and small molecule drugs already available that may be repurposed for targeted therapies of sepsis. We continue to work to establish this potential in collaboration with our colleagues at multiple hospitals and other research institutes.