A new study in Cell Reports details how a protein previously associated with regulating metabolism in the liver also plays a part in maintaining a healthy heart by ensuring that the heart wall is neither too thick nor too thin.
Scientists at Sanford Burnham Prebys and Salk Institute for Biological Studies have uncovered a new role for a protein known for its role in the brain helping control feelings of hunger or satiety, as well as in the liver to aid the body in maintaining a balance of energy during fasting. The new study shows that this protein also supports the maintenance of heart structure and function, but when it is overactive it causes thickening of the heart muscle, which is associated with heart disease.
Excessive thickening of the heart muscle—known as cardiac hypertrophy—is often the result of the heart trying to maintain proper blood flow while adapting to changes caused by other heart diseases such as hypertension or heart valve malfunction. Hypertrophy in the heart’s left ventricle affects as many as half of all patients diagnosed with type 2 diabetes, and the thickening of this chamber is known to lead to more adverse cardiovascular events such as heart attacks, strokes and sudden cardiac deaths.
“We’re interested in this because heart disease is the leading cause of death in the industrialized world,” says Karen Ocorr, PhD, an assistant professor in the Development, Aging and Regeneration Program at Sanford Burnham Prebys. “And for most of the situations—including cardiac hypertrophy—we still don’t really know the root causes.”
Ocorr, her lab and her collaborators at the Salk Institute published results on August 1, 2024, in Cell Reports, showing that a protein called CREB-regulated transcription co-activator (CRTC) is likely one of the underlying sources of cardiac hypertrophy.
“People have done a lot to understand how CRTC works in neurons and the liver, but no one has really shown that it functions in the heart,” says Cristiana Dondi, PhD, a postdoctoral associate in the Ocorr lab and first author on the study. “We were determined to change that.”
The research team knew that CRTC interacted with an enzyme called calcineurin that had been connected to cardiac hypertrophy in prior studies. They began by creating fruit flies genetically engineered with an inactive form of the gene that carries the blueprint for CRTC.
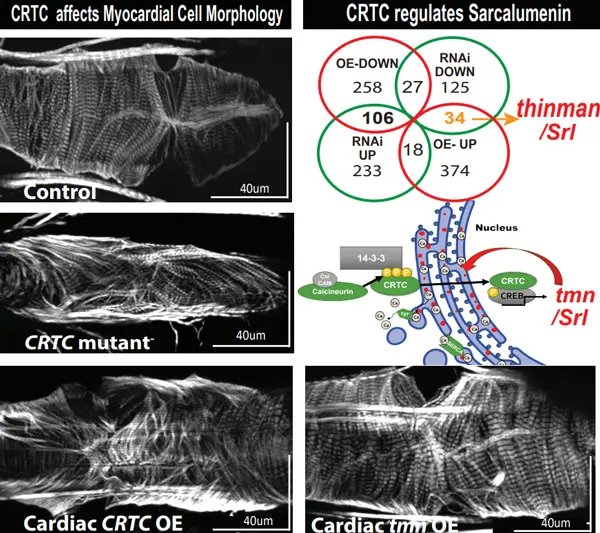
Upon testing heart rhythm with tiny electrodes and heart function with high-speed video imaging, the fruit flies without active CRTC had more difficulty recovering normal heart rhythms after experiencing stress than did normal fruit flies. The engineered flies also had thinner heart muscles with diminished function and were unable to circulate blood as effectively.
This graphical abstract uses images from the study’s experiments to highlight major findings regarding the role of a protein called CREB-regulated transcription co-activator (CRTC) in supporting the maintenance of heart structure and function, and how it is likely one of the underlying sources of cardiac hypertrophy. Image courtesy of Karen Ocorr.
“In addition to the structural defects we found in the flies with the CRTC gene systemically knocked out, we also saw an incredible amount of fibrosis,” notes Ocorr, senior author on the study. “We hardly ever see that in normal hearts, so that really struck me because fibrosis is a hallmark of heart disease.”
To make sure that these observations were specifically connected to a lack of CRTC in the heart rather than a broader developmental defect in other cells that precipitated the phenomena, the team investigated flies that only failed to make the CRTC protein in the heart while other tissues maintained their CRTC production.
“We saw very similar results to the studies with our whole-body knockout flies, so that helped us confirm that the changes were specific to a lack of CRTC in the heart,” says Dondi. To be even more sure, the group repeated the experiments eliminating CRTC only in cells surrounding the heart called pericardial cells, then in the nervous system and, finally in the fly’s fat body considered to be the fly’s equivalent of a liver. But none of these manipulations had the same effects on the heart, adding more weight to a crucial cardiac role for CRTC.
In addition to examining the effects of an absence of CRTC in the heart, the team also investigated what would happen if the heart produced too much CRTC, which is known as overexpression.
“It seems to be two sides of a structural coin,” adds Ocorr. “Without CRTC, the muscle fibers within the heart cells get disorganized. They start to have big gaps between them and then the ability of the heart to contract is reduced.”
“If you overexpress CRTC, you get the opposite. You get a lot more of the proteins than is normally needed for the heart and that’s what makes it bigger and causes hypertrophy. Although the heart is larger, it becomes too musclebound and does not function as well as a normal heart.”
In addition to finding a new agent responsible for cardiac hypertrophy alongside the well-established calcineurin enzyme, Dondi, Ocorr, and their collaborators discovered a protein whose production is controlled by CRTC and likely contributes to the heart defects observed in this publication.
“We checked how genes worked differently in the hearts with too little or too much CRTC activity,” says Dondi. “After filtering for those present in heart cells, we narrowed the list to 15 genes.”
One of these 15 genes is the fruit fly’s equivalent of the human gene Sarcalumenin which was found to be more active when CRTC was overexpressed and less active when CRTC was silenced. When the researchers prevented the production of this protein, they observed similar effects on heart structure and function as in the earlier experiments centered on CRTC. Because the loss of this protein caused hearts to be thinner the team proposed naming this gene “thinman.”
“This was another piece of evidence that CRTC controls the production of ‘thinman’ in flies, and probably also Sarcalumenin in humans, during normal heart maintenance,” says Ocorr. “The thinman gene contains instructions for proteins involved in managing calcium levels in skeletal and heart muscle cells. If something happens to CRTC, then you lose Sarcalumenin or thinman and you get calcium overload and muscle disorganization. Our studies show the CRTC-thinman connection is a novel pathway in cardiac hypertrophy, which is a very exciting new discovery in the field of heart research.”
“This finding opens a lot of possibilities for learning more about these signaling molecules and using them as targets for drugs to treat heart conditions. Sarcalumenin also has been linked to muscular dystrophy, so we see many opportunities for expanding on this work to find potential treatments for other conditions in addition to heart disease.”
Additional authors on the study include Georg Vogler, Anjali Gupta, Stanley Walls, Anaïs Kervedec, James Marchant, Michaela R. Romero, Soda Diop, Jason Goode, John B. Thomas, Alex Colas, Rolf Bodmer and Marc Montminy.
The study was supported by the National Institutes of Health, the American Heart Association, the Leona M. and Harry B. Helmsley Charitable Trust, the Clayton Foundation for Medical Research and the Kieckhefer Foundation.
The study’s DOI is 10.1016/j.celrep.2024.114549.
