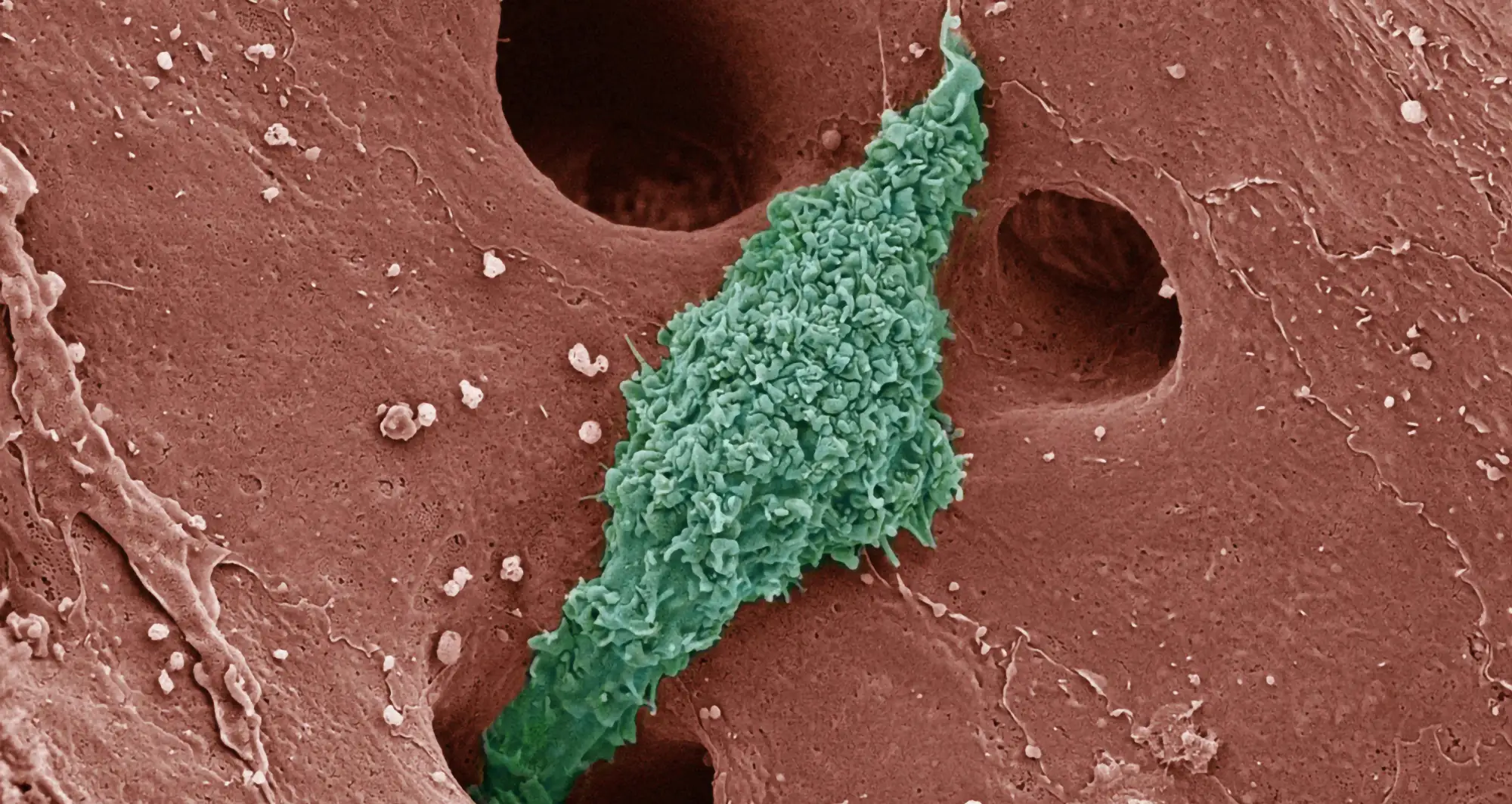
The white blood cells’ typical role is to promote inflammation and stimulate the immune response, but researchers say some actually appear to temper inflammatory conditions and improve healing.
Formerly known as nonalcoholic steatohepatitis, metabolic dysfunction-associated steatohepatitis (MASH) is an inflammatory disease characterized by liver scarring or fibrosis that progressively impairs liver function.
It is a major risk factor for cirrhosis and liver cancer. And because treatment options are limited, MASH is the most common liver disease in the United States and the second leading cause for liver transplants in the United States after cirrhosis caused by chronic hepatitis C infection.
A better understanding of the pathological processes that drive MASH is critical to creating effective treatments. In a new paper published August 19, 2024 in PNAS, a team of scientists from Sanford Burnham Prebys, the University of California San Diego School of Medicine and elsewhere, describe the complex interplay between diseased liver cells and macrophages — a type of white blood cell whose jobs include killing and removing harmful cells and pathogens and helping to spur normal healing.
Debanjan Dhar, PhD, associate professor in the Cancer Genome and Epigenetics Program at Sanford Burnham Prebys, is senior author of the study. David Brenner, MD, president and CEO of Sanford Burnham Prebys, and Christopher Glass, MD, PhD, professor of cellular and molecular medicine at UC San Diego, are corresponding authors. Souradipta Ganguly, PhD, a staff scientist at UC San Diego and Sanford Burnham Prebys, is first author.
The researchers found that the heterogeneous mix of macrophages involved with MASH was different, depending on whether the disease was progressing or regressing. More importantly, they identified specific macrophage subpopulations that are critical for resolving MASH and liver fibrosis in which accumulating scar tissue impairs the organ’s ability to function or repair itself. These fibrotic bands restrict blood flow, imperiling the entire organ.
“In MASH, Kupffer cells (a type of macrophage that resides in the healthy liver) are lost and replaced by four distinct macrophage subpopulations. When the disease is in regression — that is, the severity is decreasing —lipid associated macrophage subpopulations are dominant and express TREM2, a cell receptor that regulates cell survival, proliferation and anti-inflammatory responses,” said Brenner.
“MASH regression occurs efficiently in the presence if TREM2+ macrophages. The TREM2 molecule not only restricts the progression of MASH-fibrosis, but effectively slows it and reduce inflammation. The absence of TREM2+ macrophages worsens the disease.”

David Brenner, MD
In early and moderate stages, MASH often produces no tell-tale symptoms, which is part of the why it has reached epidemic proportions in the U.S. The American Liver Foundation estimates 80 to 100 million Americans have fatty liver disease which, undiagnosed and untreated, progresses to nonalcoholic steatohepatitis, MASH, cirrhosis, liver cancer and death, often in combination with other conditions, such as obesity.
An estimated 1.5% to 6.5% of U.S. adults have MASH, and roughly 24% of adults have metabolic dysfunction-associated steatotic fatty liver disease (MASLD), the starting point for MASH, cirrhosis and worse.

Debanjan Dhar, PhD
“Our findings suggest that TREM2 signaling is required both for the emergence of lipid associated macrophages and for their reparative functions,” said Dhar.
“Effective degradation of scar tissue as a protective mechanism is mediated by TREM2, and the absence of TREM2+ macrophages not only disrupts the liver’s ability to remove fibrotic tissue, but it also harms the entire immune response and healing process.”
“Moreover, Trem2+ macrophages can cross-talk with other types of liver cells such as hepatocytes, and hepatic stellate cells and this interaction helps to lower the hepatic fat content, promoting a healthier liver during MASH resolution”, explained Ganguly.
Going forward, the scientists say a TREM2 agonist — a drug or substance that mimics the function of TREM2 — might be beneficial for MASH/fibrosis therapy and help spur MASH and fibrosis regression in patients also undergoing lifestyle modification, weight loss or bariatric surgery.
“There is only one approved treatment for MASH, and it was only approved earlier this year,” said Glass. “Any opportunities to expand clinical options that benefit patients need to be thoroughly pursued because liver disease in this country — and around the world — is only getting worse.”
Additional authors on the study include Sara Brin Rosenthal, Kei Ishizuka, Theresa V. Rohm, Naser Khader, Sebastiano Archilei, Jerrold M. Olefsky, Ariel E. Feldstein, Tatiana Kisseleva and Rohit Loomba, all at UC San Diego; Ty D. Troutman, UC San Diego and Cincinnati Children’s Hospital Medical Center, and German Aleman Muench, Yasuyo Sano and Pejman Soroosh, Janssen Research & Development, San Diego.
This study was supported by National Institutes of Health grants to D.D. (R01DK137061, R01DK133930), Altman Clinical and Translational Research Institute (ACTRI – KL2TR001444) and the San Diego Digestive Diseases Research Center (NIH DK120515). It was partially supported by the ACTRI (NIH UL1TR001442). T.K. was supported by NIH grants DK099205, AA028550, DK101737, AA011999, DK120515, AA029019, DK091183; C.K.G by NIH grants DK091183 and HL147835. T.D.T. was supported by NIH grants P30DK063491, T32DK007044, P30DK078392, the American Association for the Study of Liver Diseases (PNC23-216751) and the Center for Inflammation and Tolerance through the Cincinnati Children’s Research Foundation. R.L. received funding support from NCATS (5UL1TR001442), NIDDK (U01DK061734, U01DK130190, R01DK106419, R01DK121378, R01DK124318, P30DK120515), NHLBI (P01HL147835), the John C. Martin Foundation (RP124). J.M.O was supported by the Diabetes Research Center (P30DK063491) and Horton JPI MRA: Obesity and its metabolic complications (20175015). A.E.F was supported by the NIH grant R01DK113592. T.V.R was supported by grants from the Swiss National Science Foundation (P2BSP3_200177) and the Larry L. Hillblom Foundation (2023-D-012-FEL).
The study’s DOI is 10.1073/pnas.240574612.