No treatments currently exist for the common condition that causes progressive vision loss
Scientists at Sanford Burnham Prebys Medical Discovery Institute have shown that the blood protein vitronectin is a promising drug target for dry age-related macular degeneration (AMD), a leading cause of vision loss in Americans 60 years of age and older. The study, published in the Proceedings of the National Academy of Sciences (PNAS), also holds implications for Alzheimer’s and heart disease, which are linked to vitronectin.
“Our findings suggest that vitronectin, which is shaped like a sticky propeller, orchestrates the formation of the spherical deposits that accumulate and cause dry AMD,” says Francesca Marassi, PhD, director of the Cancer, Molecules and Structures Programat Sanford Burnham Prebys and senior author of the study. “With this information, we can look for drugs that prevent the deposits from forming and help people retain their sight for as long as possible.”
More than 11 million Americans have AMD; and this number is expected to double by 2050 as the U.S. population ages. Of the two types of macular degeneration—wet and dry—the dry form is the most common, making up approximately 80 to 90% of cases. While the progression of dry AMD can be slowed with lifestyle changes, such as taking vitamin supplements, eating healthy, and not smoking, no pharmaceutical treatment exists.
New insights into deposit formation
Dry AMD is caused by the progressive accumulation of drusen, pebble-like deposits at the back of the eye, which results in blurry vision and, over time, vision loss. While scientists knew that these deposits contain cholesterol, fats (lipids), proteins such as vitronectin, and a mineralized form of calcium phosphate called hydroxyapatite—the same material that forms teeth and bone—how these deposits form was unknown.
Francesca Marassi used the structure of vitronectin—which is shaped like a sticky propeller—to prove that the top propeller tightly clasps calcium and hydroxyapatite, the same material that forms teeth and bones.
In the study, Marassi and her team used the structure of vitronectin—which was solved by Marassi last year—and various sophisticated biophysical tools to prove that the propeller top tightly clasps calcium and hydroxyapatite. These findings suggest a mechanism by which vitronectin drives the formation of the abnormal deposits—and reveal how this process might be interrupted.
“We know that these deposits have a cholesterol-rich lipid core that is surrounded by a shell of hydroxyapatite and a final topcoat of vitronectin,” says Marassi. “Our study suggests that vitronectin brings all these pieces together in one place to build up this complex assembly. With this information, we can start to figure out how to disrupt these interactions and break up this deposit.”
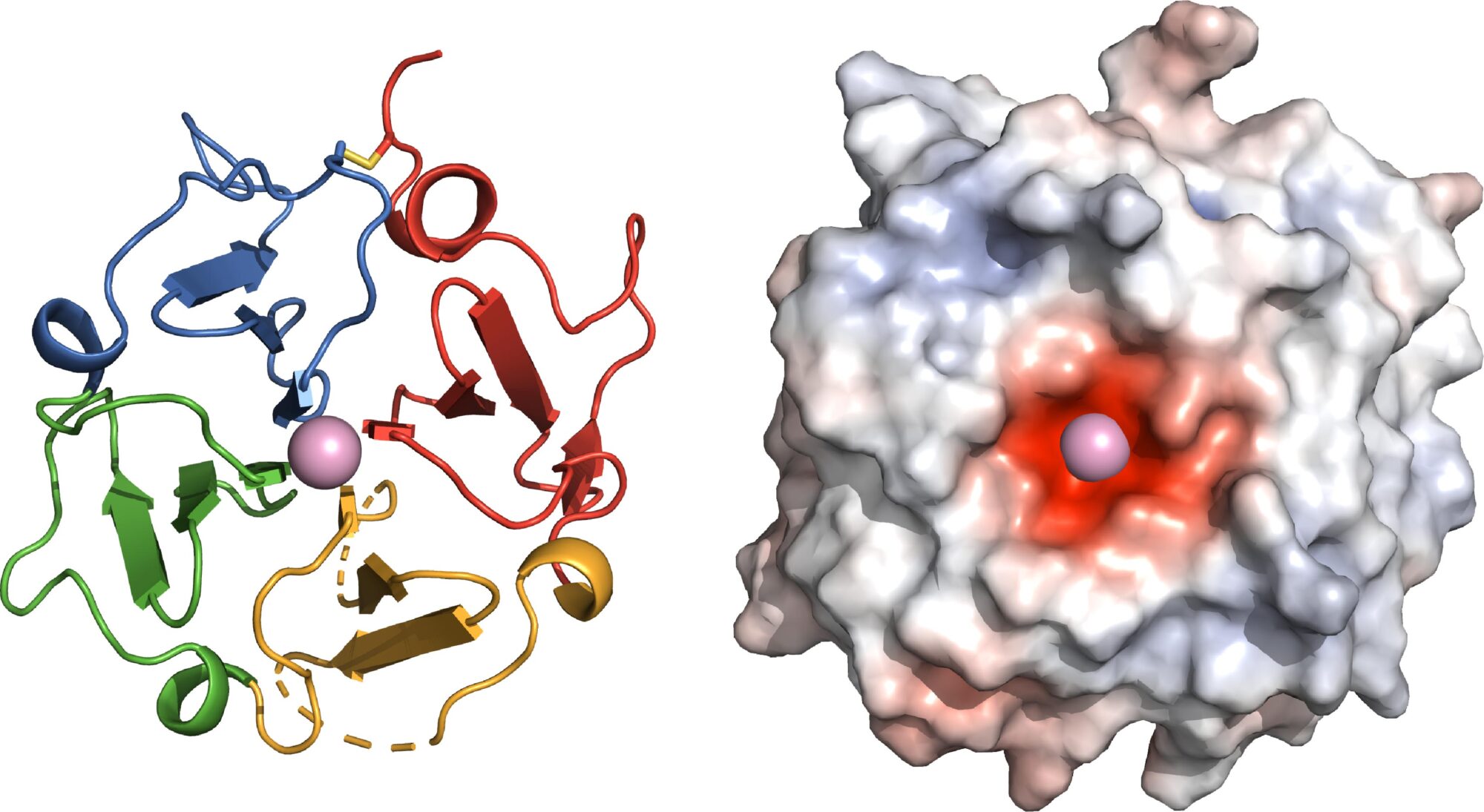
Left: The functional site of Vitronectin is shaped as a four-bladed propeller, yielding new insights into its function in the body. Right: A negatively charged center (red) allows Vitronectin to clasp a positively charged calcium atom. ©SBP
Forging ahead to drug development
Marassi is already working with scientists at the Institute’s Conrad Prebys Center for Chemical Genomics to identify vitronectin-targeting compounds that can stop drusen from forming. This drug candidate would hold promise as a treatment that slows the progression of dry AMD and potentially other plaque-related conditions. Vitronectin is also a major component of the amyloid plaques linked to Alzheimer’s disease and the cholesterol-rich plaques that cause heart disease.
“For diseases such as dry AMD and Alzheimer’s that have no effective treatment, the need for innovative science is painfully clear,” says Diane Bovenkamp, PhD, vice president of Scientific Affairs at BrightFocus Foundation, a nonprofit that advances research on macular degeneration, Alzheimer’s and glaucoma. “We are hopeful that these findings on how disease-associated proteins bind together will help scientists to design better drugs that could lead to treatments for one or more of these diseases with unmet clinical needs.”
This work was supported by the National Institutes of Health (GM118186, P30CA030199) and the Canadian Institutes of Health Research. The study’s DOI is 10.1073/pnas.2007699117.
The first author of the study is Kyungsoo Shin of Sanford Burnham Prebys. Additional study authors include James E. Kent, Chandan Singh, Lynn M. Fujimoto, Jinghua Yu and Ye Tian of Sanford Burnham Prebys; and Wonpil Im of Lehigh University.
