Alexander Strongin earned his PhD from Moscow State University in Russia in 1972 and his D.Sci. degree from the Institute of Microbial Genetics in Moscow in 1983. From 1982 to 1988, Dr. Strongin was head of the Laboratory of Functional Enzymology at the Institute of Genetics of Microorganisms in Moscow. He served as head of the Department of Biotechnology and Laboratory of Protein Engineering, Institute of Molecular Genetics, Russian Academy of Sciences, Moscow, from 1988 to 1990. From 1990 to 1994, he was a visiting professor of biochemistry in the Division of Dermatology at Washington University School of Medicine, St. Louis, Missouri. Dr. Strongin has worked in the La Jolla area since 1994, as senior staff scientist in the Biology Division at General Atomics, 1994-1995, and as senior staff scientist at the La Jolla Institute for Experimental Medicine, 1995-1999. Dr. Strongin joined Sanford Burnham Prebys on September 1, 1999.
Related Disease: Breast Cancer
William Stallcup earned his PhD in biochemistry from the University of California at Berkeley in 1972. He did postdoctoral work at the Salk Institute, where he was appointed Assistant Professor in 1976. Dr. Stallcup was recruited to Sanford Burnham Prebys in 1984.
Related Disease
Atherosclerosis, Brain Cancer, Breast Cancer, Multiple Sclerosis, Obesity, Skin Cancer and Melanoma
Molecules that control cell proliferation and motility are very important for both normal development and the pathology of many diseases. During development, immature precursor cells must undergo an extensive program of cell division in order to generate the millions of cells required to form a mature organism. In many cases these cells must also be able to migrate long distances to reach their final resting spots in the body. In diseases such as cancer, the mechanisms regulating both cell proliferation and migration are disturbed so that cells divide and migrate in an uncontrolled manner. Dr. Stallcup’s laboratory studies a cell surface protein called NG2 that appears to be involved in both cell proliferation and motility. During normal development, NG2 is found on immature cells that are actively dividing and migrating in tissues such as the brain and vasculature. When cells in these tissues mature, they no longer produce NG2. However, when cells become injured or cancerous, they once again produce NG2 and re-acquire the ability to divide and migrate. Work in the Stallcup lab is aimed at understanding the regulation of NG2 so that cell proliferation and motility can be controlled in pathological situations.
William Stallcup’s Research Report
Cell Surface Molecules in the Developing Nervous System
The NG2 chondroitin sulfate proteoglycan is a membrane-spanning protein expressed by several types of immature progenitor cells, including oligodendrocyte progenitors, chondroblasts, skeletal muscle myoblasts, smooth muscle cells, and pericytes. NG2 is also expressed by several types of highly malignant neoplasms, including melanomas, glioblastomas, and lymphomas (Burg et al, 1998). Both the progenitor cells and the tumor cells are mitotic and in some cases highly motile. There is evidence to suggest that NG2 plays a role in both growth control and motility during the development of these cell types. We are currently investigating systems in which NG2 appears to be involved in the signaling mechanisms that control these processes. We have identified three classes of signal transduction mechanisms that may be mediated or modulated by NG2.
NG2 is required for optimal activation of the PDGF alpha receptor by PDGF-AA. NG2-positive smooth muscle cells migrate and proliferate well in response to both PDGF-AA and PDGF-BB, while NG2-negative smooth muscle cells (derived from NG2 knockout mice) respond only to PDGF-BB (Grako et al, 1999). Since we can show that the alpha receptor does not undergo autophosphorylation in response to PDGF-AA, we believe the defect in the NG2-negative cells must lie at the level of receptor activation. We have now demonstrated that NG2 is capable of binding PDGF-AA (but not PDGF-BB) with fairly high affinity, and thus may participate in sequestering the growth factor or in presenting it to the signaling receptor (Goretzki et al, 1999). In this case, therefore, we believe that NG2 plays an auxilliary role to the actual signal transducing molecule.
NG2-positive smooth muscle cells and several NG2-positive cell lines also proliferate and migrate well in response to soluble type VI collagen, an extracellular matrix ligand for the proteoglycan. In contrast, the NG2-negative counterparts of these cells respond much less effectively. We have preliminary indications that the response to soluble type VI collagen involves activation of the MAP kinases Erk-1 and 2, similar to what is seen during stimulation with growth factors. Further experiments will be required to elucidate the details of this signaling cascade and to determine whether NG2 is involved directly or indirectly in activating this pathway.
The third signaling mechanism is observed upon engagement of NG2 by the substratum, a process that results in cell spreading and migration. Importantly, spreading and migration do not occur with cells expressing NG2 variants lacking the cytoplasmic domain of the proteoglycan, suggesting that interaction of NG2 with cytoplasmic ligands is required for these processes to take place. Analysis of the cytoskeletal rearrangements taking place in spreading cells reveals the extension of both filopodia and lamellipodia in response to NG2 engagement (Figure 1). These two processes are thought to be controlled by activation of the rho family GTPases cdc42 and rac, respectively, suggesting that NG2 engagement triggers activation of these GTPases. We are currently investigating the details of the signaling cascades involved in these phenomena, as well as attempting to identify cytoplasmic binding partners for NG2 which may serve as effector molecules for activation of downstream signaling events.
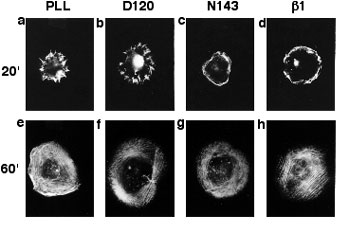
Organization of Actin in Spreading U251 Transfectants
Wild type NG2 transfectants were allowed to spread for 20 (a-d) or 60 (e-f) minutes on surfaces coated with PLL, mAb D120, mAb N143, or mAb beta(1). Cells were then fixed with two percent paraformaldehyde and stained with rhodamine phalloidin to allow visualization of filamentous actin. Radial actin spikes characteristic of filopodia were seen on PLL and mAb D120, while cortical actin bundles characteristic of membrane ruffles were seen on mAb N143 and mAb beta(1). After 60 minutes, stress fibers were apparent in all cases.
Arnold C. Satterthwait earned his PhD In Biochemistry with William Jencks from Brandeis University in 1973. He carried out postdoctoral research in Chemistry at Harvard University with Frank Westheimer, Imperial College with Alan Fersht and MIT with the Nobel laureate Gobind Khorana. In 1984, he joined The Scripps Research Institute in La Jolla, CA as an Assistant Professor. He moved to Sanford Burnham Prebys in 1998.
Related Disease
Anthrax, Breast Cancer, Cancer, HIV/AIDS, Prostate Cancer
The development of diagnostic reagents, drugs and vaccines is the visible outcome of a long process that spans the researcher’s laboratory and doctor’s office. The translation of disease discoveries into early detection, treatment, and prevention both tests and shapes our understanding of disease. Traditionally, drug companies have screened large collections of compounds against diseases to identify drugs. The Satterthwait lab seeks to take advantage of the explosion of new discoveries at the molecular level. We have developed synthetic methods that allow us to independently make and manipulate the critical three-dimensional regions of proteins that are being implicated in many diseases. These mini proteins are being used to assess new theories of disease at the molecular level to identify targets for various uses. We are currently using mini proteins to identify new antibodies (HIV-1), cancer drugs (prostate, breast and lung), and vaccines (anthrax).
Arnold Satterthwait’s Research Report
Peptide engineering relies on synthetic procedures to fold peptides into bioactive structures. It seeks to bridge a gap between chemistry and molecular biology by reducing the active sites of proteins to smaller molecules. Although synthetic peptides show occasional activity they are, unlike proteins, disordered and because of this often inactive. By refolding peptides into three-dimensional structures, they become active, opening up new avenues for studies on protein structure and function as well as providing leads for drugs and vaccines.
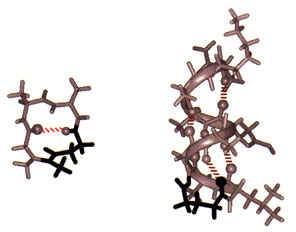
Solid-phase synthesis of a peptide constrained with a hydrazone covalent hydrogen bond mimic. The NMR structure of Sar-Ala-Ala-Gly (left) stabilized as a Type I turn (10% of protein structure) with an amidinium link (in black). The NMR structure of acetyl-Gly-Leu- Ala- Gly-Ala-Glu-Ala-Ala-Lys-Ala-amide (right) stabilized as an a-helix at its N-terminus with a hydrazone link (in black).
To fold peptides, we developed covalent hydrogen bond mimics. On average, greater than 60% of the amino acids in globular proteins engage in main-chain to main-chain hydrogen bonding (NH –> O=CRNH). In addition, protein substructures are defined by distinct hydrogen bonding patterns. Because hydrogen bonds are weak bonds, insufficient for stabilizing peptide structure, we replace them at structure defining positions in peptides with amidinium (N-C(R) = NH-CH2CH2) and hydrazone (N-N=CH-CH2CH2) covalent links. To simplify these transformations, we developed machine-assisted, multiple-peptide-synthesis procedures for inserting the hydrazone link into peptides which we link to automated multiple purifications. While these procedures, like peptide synthesis, remain labor intensive and often problematic, the critical problems have been breached.
Conformational analysis is as much a part of peptide engineering as synthesis because any claim to structure requires rigorous proof and further advances rely on understanding the relation between structure and chemistry. We have made considerable use of 2D NMR spectroscopy for structural analysis and calculations and with the help of collaborators, X-ray crystallography. These studies show unequivocally that covalent hydrogen bond mimics can stabilize peptides as b-turns, the a-helix, and even complex loops, which together make up the majority of protein substructures found on the surfaces of globular proteins.
Because protein substructure mimetics are now accessible, we have been examining the relationship between structure and activity by comparing the activities of peptides with substructure mimetics. From the several examples we have studied in detail, it is clear that remarkable gains in activity can be achieved.
Erkki Ruoslahti earned his MD and PhD from the University of Helsinki in Finland in 1967. After postdoctoral training at the California Institute of Technology, he held various academic appointments with the University of Helsinki and the University of Turku in Finland and City of Hope National Medical Center in Duarte, California. He joined Sanford Burnham Prebys in 1979 and served as its President from 1989-2002. He was a Distinguished Professor at University of California Santa Barbara in Biological Sciences 2005-2015. His honors include elected membership to the U.S. National Academy of Sciences, National Academy of Medicine, American Academy of Arts and Sciences, and the European Molecular Biology Organization, the Japan Prize, Gairdner Foundation International Award, G.H.A. Clowes Award, Robert J. and Claire Pasarow Foundation Award, and Jacobaeus International Prize. He was a Nobel Fellow at the Karolinska Institute in Stockholm in 1995, and is an Honorary Doctor of Medicine from the University of Lund, as well as a Knight and Commander of the Orders of the White Rose the the Lion of Finland. In 2022, Dr. Ruoslahti was announced as one of three winners of the Albert Lasker Basic Medical Research Award.
Education
1966: MD, University of Helsinki in Finland
1967: PhD, University of Helsinki in Finland
Awards and Honors
2022: Albert Lasker Award for Basic Medical Research
Commander of the Order of the Lion of Finland
Knight of the Order of the White Rose of Finland
2012: Thomson Reuters Citation Laureate
2005: Japan Prize in Cell Biology
2003: Jubilee Lecturer, Biochemical Society
1998: Jacobaeus International Prize
1997: Gairdner Foundation International Award
1995: Nobel Fellow at the Karolinska Institutet in Stockholm
1991: Honorary doctorate in medicine from Lund University, Sweden
1990: American Association for Cancer Research – G.H.A. Clowes Memorial Award
Member
National Academy of Sciences
National Academy of Medicine
American Academy of Arts and Sciences
European Molecular Biology Organization
Related Disease
Alzheimer’s Disease, Atherosclerosis, Brain Cancer, Breast Cancer, Cancer, Prostate Cancer
The Ruoslahti laboratory studies peptides that home to specific targets in the body, such as tumors, atherosclerotic plaques and injured tissues. These peptides, which usually bind to receptors in the vessels of the target tissue, can be used to selectively deliver diagnostic probes and drugs to the target. The latest development is the discovery of homing peptides with tumor-penetrating properties. The CendR tissue penetration pathway is a new endocytosis/trans-tissue transport pathway (Pang et al., Nat Comm. 2014). The current focus is on enhancing the effects of coupled and co-injected drugs with the tumor-homing peptides, particularly in mouse models of breast cancer and glioblastoma. This laboratory also studies the receptors for the peptides and the mechanism of their tumor penetration activity.
Erkki Ruoslahti’s Research Report
Dr. Ruoslahti’s main scientific contributions are in the field of cell adhesion. He was one of the discoverers of fibronectin. His laboratory subsequently discovered the RGD cell attachment sequence in fibronectin and isolated RGD-directed cellular receptors, now known as integrins. The RGD discovery has led to the development of drugs for diseases ranging from vascular thrombosis to cancer.
Dr. Ruoslahti current studies deal with peptides that specifically target a diseased tissue, particularly its blood vessels. The peptides can be used to deliver drugs and nanoparticles to sites of disease, such as a tumor. The molecules targeted by such disease-specific peptides are of interest regarding their possible role in the disease and potential targets for drug development.
Vascular Zip Codes
The Ruoslahti laboratory screens large collections (“libraries”) of random peptides to identify those that bind to specific targets in tissues. The peptides in the library are displayed on the surface of phage (a virus that infects bacteria), and the screening is done in vivo. When the library is injected into the circulation of a mouse, phage particles that display peptides capable of binding to a selected target tissue, such as a tumor, accumulate at the target where they can be collected and their peptide identified. The process primarily probes the vasculature of the target tissue, unless the vasculature is very leaky. The method has revealed a wealth of specific features, or “vascular zip codes”, in the vessels of individual tissues and tumors. Peptides that specifically home to tumors because they recognize angiogenesis-associated or tumor-type specific markers in tumor blood vessels and can even distinguish the vessels of pre-malignant lesions from those of fully malignant tumors. Homing peptides have also revealed a zip code system of molecular changes in tumor lymphatics.
Synthetic homing peptides have been used to target drugs, biologicals, and nanoparticles into tumors. The targeting can increase the efficacy of a drug while reducing its side effects. Even a non-specifically toxic compound can be converted into a compound that selectively affects the targeted tissue. The peptides make it possible to identify the target molecules (receptors) for the peptides. The receptors of tumor-homing peptides often play a functionally important role in tumor vasculature, and because of this are candidates for drug development.
Tumor-penetrating Peptides
A few years ago the laboratory discovered peptides that not only home to tumor vessels, but are transported through the vascular wall and deep into tumor tissue. The key feature of these peptides is a R/KXXR/K sequence motif, named C-end Rule (CendR) motif or element. In tumor-penetrating peptides, the CendR element is cryptic. These peptides penetrate into tumor tissue in a 3-step process: (i) The peptide binds to a primary receptor on tumor endothelium. In iRGD, the RGD motif recognizes the avb3/avb5 integrins; the primary receptor for the LyP-1 family of peptides is cell surface p32/gC1qR. (ii) The peptide is then cleaved by a protease to expose the CendR element at the C-terminus of the peptide; and, (iii) the CendR element mediates binding to neuropilin-1 (NRP-1), to induce vascular and tissue penetration. The CendR transport pathway triggered through NRP-1 resembles macropinocytosis, but differs from it in being receptor-mediated. Importantly, the responsiveness of the pathway to triggering through NRP-1 is regulated by the nutrient status of cells and tissues. Its physiological function is likely to be to transport nutrients into tissues that lack them. Our ability to trigger the pathway specifically in tumors makes it useful in delivering drugs into tumors.
Targeting the Brain
The Ruoslahti laboratory has recently also applied phage screening to the identification of peptides that target brain diseases. So far, a peptide that specifically recognizes sites of brain injury, and a panel of peptides that are specific for Alzheimer’s brain have been obtained. This topic will be an expanding focus of the laboratory in the near future.
Nanomedicine
A major focus is to use homing peptides as targeting elements to deliver nanoparticles into tumors and other sites of disease. Nanoparticles are considered a promising new approach in medicine because they can be designed to perform more functions than a simple drug. The vasculature is an excellent target for nanoparticles because tumor vessels are readily available for circulating particles. In collaboration with chemistry and bioengineering laboratories, multifunctional nanoparticles for tumor targeting have been constructed. These particles can be directed into tumors in a highly selective manner as demonstrated by histology, non-invasive imaging, and tumor treatment results. The laboratory has constructed nanoparticles with the ability to amplify their own homing to tumors, and are currently working on nanoparticles coated with tumor-penetrating peptides. More recent work has dealt with nanoparticles that target brain diseases or atherosclerotic plaques. The general goal is to engineer nanoparticles with multiple functions. In addition to the specific targeting, such functions include avoidance of the reticuloendothelial system, self-amplification of the targeting, exit from vessels into tissue, ability to send signals for imaging, and controlled drug delivery.
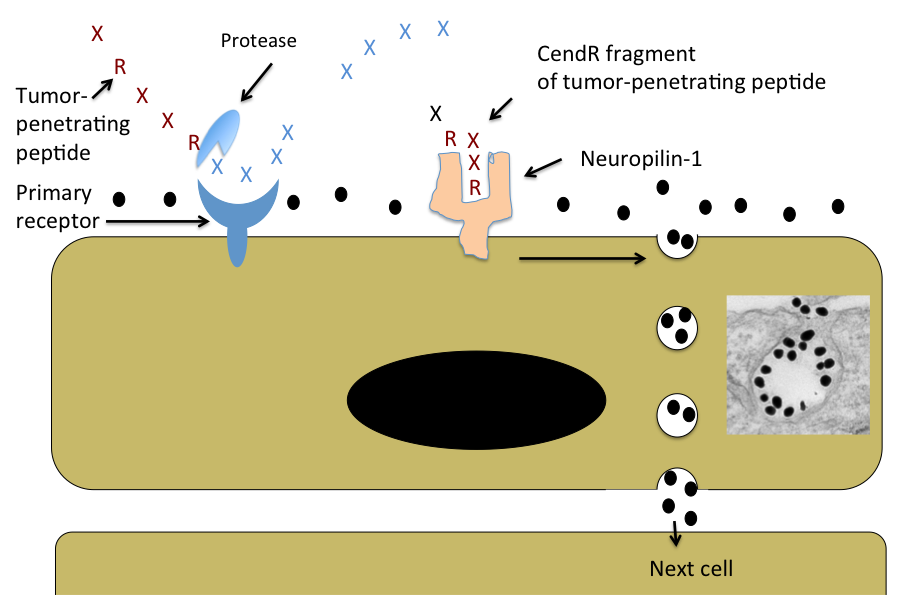
Schematic Representation of the CendR Trans-tissue Transport Pathway
Note that CendR effect enhances the tissue penetration of molecules (depicted here as a black dots) that are co-administered with the peptide, as well as of cargo coupled to the peptide. The inset shows an electron microscopic image of a CendR endocytic vesicle that is budding from the cell surface into the cytoplasm and contains CendR peptide-coated gold nanoparticles (dark dots) See Ruoslahti, Adv. Drug Deliv. Rev. 2016.
Reviews
Ruoslahti E. Tumor penetrating peptides for improved drug delivery. Adv Drug Deliv Rev. 2016 Apr 1. pii: S0169-409X(16)30094-1. doi: 10.1016/j.addr.2016.03.008. Review. PMID: 27040947
Ruoslahti, E. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv. Mat. (review article) 24:3747-3756. (2012). PMCID: PMC3947925
Robert Oshima graduated from the University of California Santa Barbara in Cellular Biology. He earned his PhD from the University of California at San Diego in 1973 with Paul Price. He joined Dr. Jerry Schneider’s laboratory in the UCSD Medical School to work on the biochemistry of cystinosis, a genetic lysosomal storage disease. During that time, he contributed to the development of a treatment that extends the life of patients greatly.
He acquired expertise in developmental biology and stem cells in the laboratories of Drs. Boris Ephrussi and Mary Weiss at the Centre National Recherche Scientifique, Gif-sur-Yvette, France in 1975. He continued those studies upon returning to UCSD and then moved to MIT in 1979 where he purified two markers of mouse stem cell differentiation that are widely used in the cancer pathology and developmental studies.
He joined the Sanford Burnham Prebys (formerly known as the La Jolla Cancer Research Foundation) in 1982 where he acted as Associate Scientific Director, a Program Director in the NCI designated Cancer Center, Postdoctoral Training Program Director, started the Tumor Analysis Shared Service and directed research on stem cells and cancer that resulted in over 100 publications. He also served as a reviewer for multiple cancer research granting agencies and taught at UC San Diego as an Adjunct Professor of Pathology from 1997.
He is currently Professor Emeritus (2015) and continues to advise and consult in cancer research. His particular cancer research interest is in methods of directing premalignant cancer cells to adopt a normal benign cell fate instead of becoming invasive malignant cancer.
Related Disease
Breast Cancer, Colorectal Cancer, Crohn’s Disease (Colitis), Preeclampsia
Cancer and development are closely related processes. The Oshima laboratory investigates the differences between normal stem cells and cancer stem cells. Furthermore, we are searching for genes and chemical compounds that control the differentiation of cancer cells and persuading them to adopt a normal developmental fate. Differentiation therapy has the promise of causing fewer deleterious side effects than killing cancer cells.
Robert Oshima’s Research Report
Stem Cells in Breast Cancer and Placental Development
Ets2 is one of a family of transcription factors that all utilize a conserved Ets protein domain for DNA binding. Like its Drosophila homologs, pointed and yan, Ets2 is regulated by growth factors and oncogenes that use Ras signaling pathways. The phosphorylation of a single threonine residue in an evolutionarily conserved protein-docking domain of Ets2 results in transcriptional activation and the induction of Ets2-dependent genes.
Early mouse placental development is dependent on the function of the Ets2 transcription factor. Ets2 regulates trophoblast stem (TS) cell self-renewal and thus placental development. One of the genes regulated by Ets2 in TS cells is the Cdx2 homeobox transcription factor. In collaboration with Dr. Mana Parast at UCSD, we extended our interest in placental development by developing a model system of human placental development. Human embryonic stem cells (ESC) and induced pluripotent stem cells (IPSC) can differentiate in culture to a trophoblast like derivative. We are screening chemical libraries by high content antibody staining methods for chemicals that improve the yield of trophoblast progenitors and direct their differentiation to either extra villous, invasive trophoblast cells or synchiotrophoblast layers.
Recently, we developed methods for the selective propagation of mouse mammary cancer stem cells in culture (Castro et al. Stem Cells 2013). These cells were shown to be capable of differentiation to a benign luminal epithelial-like fate both in culture and in animals. Inhibition of ROCK1 kinase inhibited both their spontaneous differentiation to luminal epithelial cells and their adoption of a mesenchymal tumorigenic phenotype.
Previously, in collaboration with Dr. Alexey Terskikh, we investigated the role of the Maternal Embyronic Leucine Zipper Kinase (MELK) in normal mammary epithelial stem cells and mouse mammary cancer. Using a transgenic reporter gene for Melk expression, we found that Melk expression is preferentially expressed in proliferative mammary epithelial progenitor cells and tumor cells. Both tumorsphere formation in culture and tumor formation in vivo is suppressed by knocking down Melk expression with Lentiviral-mediated shRNA in MMTV-Wnt1 tumor cells. These results have recently been confirmed in human triple negative breast cancer cells.
Furthermore, the development of differentiation therapy came from a collaborative project with Pfizer. Administration of bosutinib, a Src family inhibitor, to mice with mammary tumors caused by the MMTV-PyMT oncogene greatly restricted tumor progression by inducing differentiation of the tumor to epidermal and lactational cell fates without widespread cell death. This is an example of the possibility of restricting cancer by inducing differentiation.
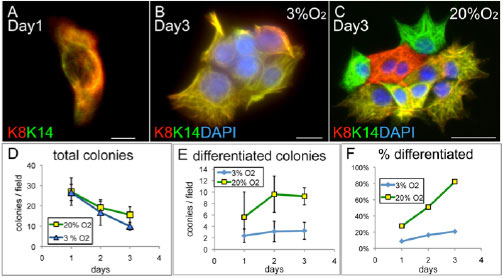
Figure 1. Regulation of cancer stem cell differentiation by low oxygen atmosphere. Single cancer stem cells were plated in two different concentrations of O2 and stained for markers of luminal (K8) or basal (K14) epithelial cells. In 20 percent O2 differentiated cells expressing only K8 or K14 are observed. (Castro et al. 2013)
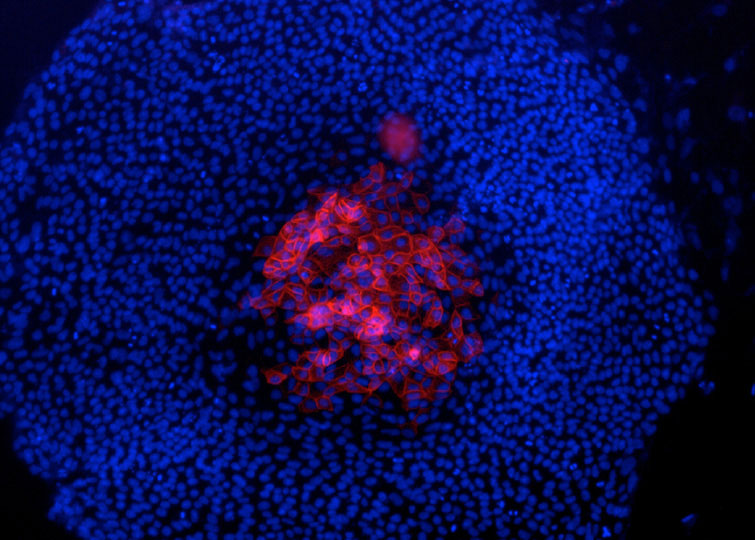
Figure 2. Presumptive trophoblast derivatives on the top of an hES cell colony, identified by keratin 7 staining (red). Nuclei stained by DAPI (blue). (Maurer et al. 2008)
Michiko N. Fukuda earned her PhD in biochemistry at the University of Tokyo in 1980. She did postdoctoral work at Fred Hutchinson Cancer Research Center in Seattle prior to her recruitment to Sanford-Burnham Medical Research Institute in 1982.
Education
1980: PhD, University of Tokyo, Biochemistry
1970: MS, University of Tokyo, Biochemistry
1968: BS, Tokyo University of Education, Botany
Related Disease
Breast Cancer, Cancer, Congenital Disorders of Glycosylation, Endometriosis, Glycosylation-Related Disorders, Inherited Disorders, Ovarian Cancer, Prostate Cancer, Testicular Cancer
Michiko Fukuda’s Research Report
Identification of Peptide that Delivers Drugs to Tumors
Chemotherapy effectiveness is often limited by drug toxicity in healthy tissues, although methods that spare normal cells by delivering drugs specifically to tumors may help to overcome this constraint. We have identified a promising tumor-targeted drug delivery vehicle known as the IF7 peptide. Using in vitro assays, we found that the IF7 peptide bound to the protein annexin 1 (Anxa1), which is known from previous studies by others to be enriched on the surface of tumor vasculature in several tumor types. When we injected a fluorescently labeled IF7 peptide into mice with tumors, fluorescent signals appeared in the tumors within one minute of injection. By contrast the tumors showed no fluorescence when mice were pre-injected with anti-Anxa1 antibodies that inhibits IF7-Anxa1 binding, suggesting that the peptide targets tumor by homing to Anxa1.
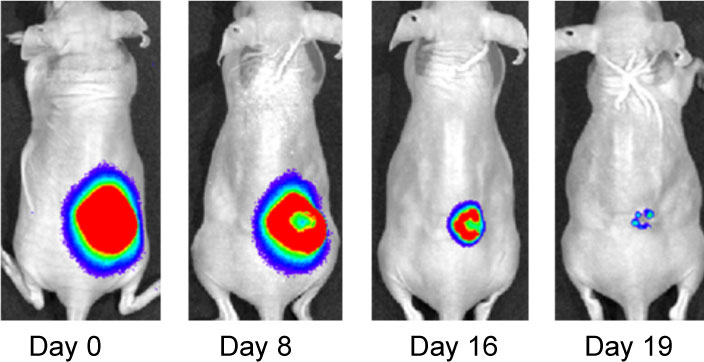
Effect of IF7-conjugated anti-cancer drug SN-38 on colon cancer mouse model. Activa cancer cells in a large tumor produce chemiluminescence which is captured by imaging. (Hatakeyama et al, 2011).
When IF7 peptide was conjugated with potent anti-cancer drug SN-38 and IF7-SN38 conjugate was injected intravenously to mice with tumors, IF7 could deliver SN-38 to tumors. We found that daily injections of an IF7-SN38 conjugate reduced a large tumor in the mouse without apparent side effects, whereas non-homing peptide-SN38 conjugate or with SN-38 alone did not reduce the tumors (Hatakeyama et al, 2011). The findings suggest that IF7 peptide may represent a clinically relevant vehicle for anti-cancer drugs.
Role of Trophinin in Human Embryo Implantation and Cancer
Invasion of the trophoblast into the endometrium, an essential element of embryo implantation, resembles invasion of malignant tumors. At the initial phase of implantation, the trophoblast and the uterine epithelium establish their first contact via their respective apical cell membranes. We have identified new molecules, trophinin, tastin, and bystin that mediate cell adhesion between trophoblastic cells and endometrial epithelial cells at the respective apical cell membranes. Trophinin is an intrinsic membrane protein, and tastin and bystin are cytoplasmic proteins. All of these molecules are strongly expressed in cells involved in embryo implantation in humans. However, trophinin is not expressed in human endometrial epithelia throughout the hormonal cycle, except only those cells located close to the implanting blastocyst. Trophinin expression by endometrial epithelia is induced by human chorionic gonadotrophin (hCG) derived from the implanting embryo (Sugihara et al, 2008). While embryos invade maternal cells (Sugihara et al, 2007), maternal tissue accepts embryos. We asked what happens in the maternal epithelia when trophinin-mediated adhesion takes place, and found that trophinin-mediated cell adhesion triggers an apoptotic signal in maternal epithelial cells (Tamura et al, 2011).
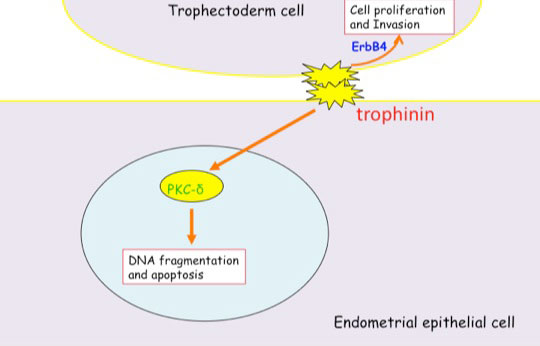
Distinct signal transduction of trophinin-mediated cell adhesion in trophectoderm cell and endometrial epithelial cells. In trophoblastic cells, ErbB4 (receptor tyrosine kinase) is arrested by bystin/trophinin complex. When trophinin-mediated cell adhesion takes palce, ErbB4 is released from bystin. This allows ErbB4 to be activated by phosphorylation. In endometrial epithelial cells, trophinin-mediated cell adhesion releases PKCd from trophinin. PKCd was then translocated to the nucleus, where it activates caspase 3 for apoptosis (Tamura et al., 2011).
References Cited
Hatakeyama S, Sugihara K, Shibata TK, Nakayama J, Akama TO, Tamura N, Wong SM, Bobkov AA, Takano Y, Ohyama C, Fukuda M, Fukuda MN (2011) Targeted drug delivery to tumor vasculature by a carbohydrate mimetic peptide. Proc Natl Acad Sci U S A 108: 19587-19592
Sugihara K, Kabir-Salmani M, Byrne J, Wolf DP, Lessey B, Iwashita M, Aoki D, Nakayama J, Fukuda MN (2008) Induction of trophinin in human endometrial surface epithelia by CGbeta and IL-1beta. FEBS Lett 582: 197-202
Sugihara K, Sugiyama D, Byrne J, Wolf DP, Lowitz KP, Kobayashi Y, Kabir-Salmani M, Nadano D, Aoki D, Nozawa S, Nakayama J, Mustelin T, Ruoslahti E, Yamaguchi N, Fukuda MN (2007) Trophoblast cell activation by trophinin ligation is implicated in human embryo implantation. Proc Natl Acad Sci U S A 104: 3799-3804
Tamura N, Sugihara K, Akama TO, Fukuda MN (2011) Trophinin-mediated cell adhesion induces apoptosis of human endometrial epithelial cells through PKC-delta. Cell Cycle 10 : 135-143
Education
University College Dublin
BSc and PhD, Pharmacology, cell and molecular biology, signal transduction and cancer, First Class Honors
Related Disease
Brain Cancer, Breast Cancer, Leukemia/Lymphoma, Lung Cancer, Pancreatic Cancer, Skin Cancer and Melanoma
Dr. Finlay’s research is focused on apoptosis (programmed cell death) and how this process can be manipulated to kill cancer cells.
A career history of fundamental discovery and translational research in immunology has guided Dr. Ware to identify new drug targets and develop novel therapeutics. Dr. Ware’s career in immunology and virology began in 1982 when he became a Professor at the University of California, Riverside’s Division of Biomedical Sciences. In 1996, he joined the La Jolla Institute for Immunology in San Diego as Head of the Division of Molecular Immunology. Professor Ware joined Sanford Burnham Prebys Medical Discovery Institute in 2010, serving as the Director of the Infectious and Inflammatory Disease Center and Adjunct Professor of Biology at the University of California at San Diego. He is currently the Director of the Laboratory of Molecular Immunology, which focuses on discovering and designing immunotherapeutics.
As an educator, he taught medical students immunology and virology. He trained over 60 postdoctoral fellows and graduate students who chose careers in research in academic and pharmaceutical science, patent law, or teaching.
Dr. Ware advises scientific panels and review boards for the National Institutes of Health and serves on the scientific advisor boards for the Allen Institute for Immunology and the Arthritis National Research Foundation. Scientific advisor with several biotechnology and pharmaceutical companies on immunotherapy for cancer and autoimmune diseases using innovative approaches to target discovery and drug development.
Dr. Ware’s research program is dedicated to unraveling the intricate intercellular communication pathways that govern immune responses. His work, which centers on cytokines in the Tumor Necrosis Factor (TNF) Superfamily, particularly those that regulate cell survival and death in response to viral pathogens, spans the domains of cancer,autoimmune and infectious diseases.
At Sanford Burnham Prebys, Dr. Ware is pivotal in promoting the translation of the faculty’s scientific discoveries. His efforts have led to the Institute’s reputation as a productive and preferred partner in collaborations with Pharma, including multi-year research and drug development projects with Eli Lilly and Avalo Therapeutics. His success translating fundamental knowledge into rational drug design has led to three novel therapeutics targeting inflammatory pathways, currently in clinical trials.
Education
1981-1982: T cell Immunology, Dana-Farber Cancer Institute of Harvard Medical School in Boston, MA. Tim Springer and Jack Strominger, advisors.
1979-1981: Biochemistry of Complement, University of Texas Health Science Center, San Antonio, TX. W Kolb, advisor
1974-1979: PhD in Molecular Biology and Biochemistry from the University of California, Irvine; Gale Granger, PhD mentor.
Honors and Recognition
Distinguished Fellow, American Association of Immunologists
Honorary Lifetime Membership Award International Cytokine and Interferon Society
Hans J. Muller-Eberhardt Memorial Lecture
Biotech All Star, San Diego Padres Awar
“Pillars of Immunology” discovery of the Lymphotoxin-b Receptor, published in Science
Outstanding Alumnus, Ayala School of Biological Sciences, University of California, Irvine
National MERIT Award R37 (10 years), National Institute of Allergy and Infectious Disease, NIH
National Research Service Award, NIH Postdoctoral Fellowship
Related Disease
Arthritis, Breast Cancer, Cancer, Crohn’s Disease (Colitis), Infectious Diseases, Inflammatory Bowel Disease, Inflammatory/Autoimmune Disease, Inherited Disorders, Leukemia/Lymphoma, Myeloma, Pathogen Invasion, Psoriasis, Systemic Lupus Erythematosus, Type 1 Diabetes
Research in the Laboratory of Molecular Immunology is directed at defining the intercellular communication pathways controlling immune responses. Our work is focused on the Tumor Necrosis Factor (TNF)-related cytokines in regulating decisions of cell survival and death, especially in responses to viral pathogens. Translational research is redirecting the communication networks of TNF superfamily to alter the course of autoimmune and infectious disease and cancer.
Carl Ware’s Research Report
Discovery of the TNF-LIGHT-LTαβ Network
The molecular elements of this cellular communication network were revealed with our discovery of Lymphotoxin-β and its formation of the trimeric heterocomplex with LTαβ1 and its signaling receptor, Lymphotoxin-β Receptor2. The LTβR revealed a new inter-cellular communication pathway that provides a key mechanism underlying the development and homeostasis of lymphoid organs. A second ligand we discovered, LIGHT (TNFSF14), is a novel ligand for the herpesvirus entry mediator (HVEM; TNFRSF14), and surprisingly, the LTβR3 heralding the concept that TNF, LTαβ, and LIGHT form an integrated signaling network thru distinct receptors controlling inflammation and host defense.4
LTβR Signaling in Host Defense and Immune Evasion
Our investigations into the mechanisms of virus evasion of the immune system revealed an essential role of the LTβR pathway in regulating the type 1 interferon response to cytomegalovirus5 and now recognized as a major defense against other pathogens. LTβR signals differentiate macrophages and stromal cells into IFN-producing cells. LTβR transcriptomics and proteomics datasets we generated revealed a novel constellation of anti-viral host defense mechanisms6. Importantly the role of the LTβR pathway to alter tissue microenvironments by differentiation of specialized stromal cells has implications for promoting effective immune responses to cancer.
Discovery of the HVEM-BTLA Immune Checkpoint
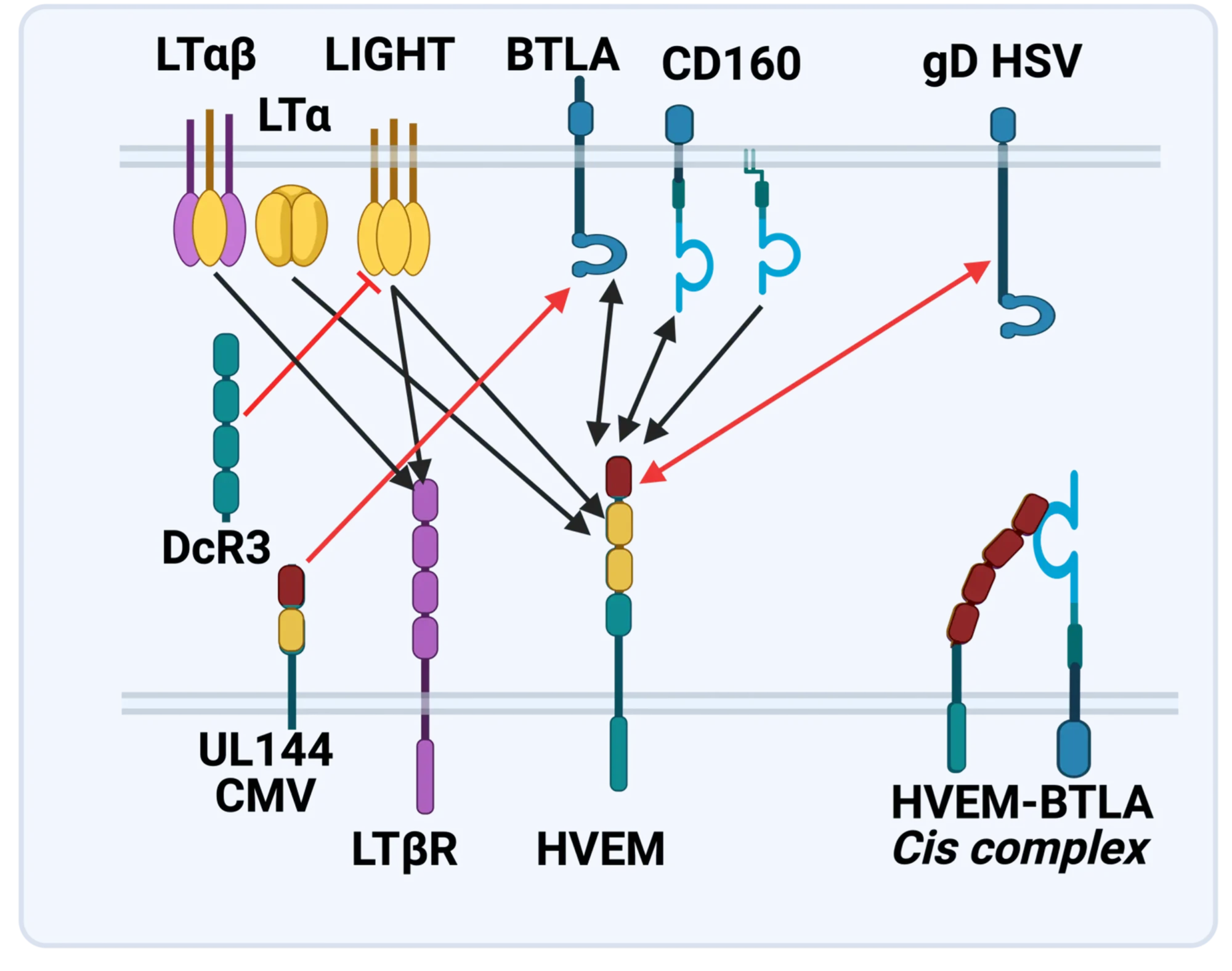
The LTBR-HVEM-BTLA Network in the TNF Superfamilies. Arrows indicate ligand-receptor binding (black), inhibitors (red arrow) and bidirectional signaling (dual arrowheads); HSV, herpes simplex virus; CMV, human cytomegalovirus. BTLA and CD160 are Ig Superfamily proteins. BTLA is an inhibitory checkpoint receptor; DcR3, decoy receptor 3 inhibits LIGHT binding to HVEM and LTBR.
The discovery that HVEM is a coreceptor for the immune checkpoint, B and T lymphocyte attenuator (BTLA), an Ig superfamily member, established a new paradigm in TNF Receptor signaling pathways 7. Additional investigations revealed the importance of the HVEM-BTLA system in limiting immune responses, including T cell help for B cell clonal expansion, antibody maturation, and secretion8. HVEM-BTLA also regulates control of the intestinal microbiome, limiting invasion of pathogenic bacteria and enhancing Treg cell homeostasis 9. The diverse roles of this pathway are seen in the loss of BTLA signaling from mutations in HVEM frequently present in B cell lymphomas10. Additional layers of immune regulators, CD160 and DcR3, control the LIGHT-HVEM-BTLA pathways, revealing this network as a key mechanism controlling immune homeostasis.
Appreciating the fundamental features of the TNF-LIGHT-LTab Network in effector and homeostasis mechanisms presents a target-rich resource for therapeutic intervention in autoimmunity, infection, and cancer11, 12.
Translational research and Immunotherapy
- 2021-current: Lead Scientific Advisor, Avalo Therapeutics
A neutralizing, fully human mAb (quisovalimab) to the proinflammatory cytokine LIGHT (TNFSF14) completed Phase I with an excellent safety profile and a Phase II trial establishing efficacy in COVID-19 pneumonia (NCT0441205)13. We identified elevated serum levels of LIGHT in hospitalized patients with COVID1914 spurring a randomized, double-blind, multicenter, proof-of-concept trial with adults hospitalized with COVID-19-associated pneumonia and mild to moderate ARDS15. The results established efficacy with a significant proportion of patients remaining alive and free of respiratory failure through day 28 after receiving quisovalimab, most pronounced in patients >60 years of age (76.5% vs. 47.1%, respectively; P = 0.042). These results and animal models validated LIGHT as a target for non-COVID inflammatory conditions, clinical trials ongoing in asthma (NCT05288504)12. - 2021-current: Principal Investigator, Avalo Therapeutics – Sanford Burnham Prebys collaboration
Bioengineered a first-in-class checkpoint agonist targeting BTLA immune checkpoint16 in preclinical development - 2019-current: Director and Principal Investigator, Fair Journey Biologics – Sanford Burnham Prebys collaboration
Immunotherapy for TNBC and PANC, in preclinical development - 2015-2022: Director and Lead Principal Investigator, LILLY-Sanford Burnham Prebys Collaboration in Autoimmunity
Collaborative research partnership with Eli Lilly involved target discovery and therapeutic development directed at immune regulators for autoimmune diseases. The collaboration produced three novel biologics currently in Phase I/2 trials (NCT03933943). The collaboration included a target discovery platform for T cell effector memory and NK cell immunomodulators. - 2015-2020: Lead Principal Investigator, Sanford Burnham Prebys – Capella Biosciences collaboration
Created a fully human mAb specific for membrane LIGHT (CBS001); phase I initiated (NCT05323110). - 2016-2020: Lead Scientific Investigator, Boehringer Ingelheim – Sanford Burnham Prebys Collaboration
Target discovery collaboration in inflammatory and fibrotic diseases6 - 2012-2014: Pfizer Innovation Center, Principal Investigator Bioengineering TNFR Superfamily in Autoimmune disease
- 1. Browning, J.L. et al. Lymphotoxin beta, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell 72,847-856 (1993).
- 2. Crowe, P.D. et al. A lymphotoxin-beta-specific receptor. Science 264,707-710 (1994).
- 3. Mauri, D.N. et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity 8,21-30 (1998).
- 4. Ward-Kavanagh, L.K., Lin, W.W., Sedy, J.R. & Ware, C.F. The TNF Receptor Superfamily in Co-stimulating and Co-inhibitory Responses. Immunity 44,1005-1019 (2016).
- 5. Schneider, K. et al. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell Host Microbe 3,67-76 (2008).
- 6. Virgen-Slane, R. et al. Cutting Edge: The RNA-Binding Protein Ewing Sarcoma Is a Novel Modulator of Lymphotoxin beta Receptor Signaling. J Immunol 204,1085-1090 (2020).
- 7. Sedy, J.R. et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol 6,90-98 (2005).
- 8. Mintz, M.A. et al. The HVEM-BTLA Axis Restrains T Cell Help to Germinal Center B Cells and Functions as a Cell-Extrinsic Suppressor in Lymphomagenesis. Immunity 51,310-323 e317 (2019).
- 9. Stienne, C. et al. Btla signaling in conventional and regulatory lymphocytes coordinately tempers humoral immunity in the intestinal mucosa. Cell reports 38,110553 (2022).
- 10. Sedy, J.R. & Ramezani-Rad, P. HVEM network signaling in cancer. Adv Cancer Res 142,145-186 (2019).
- 11. Croft, M., Benedict, C.A. & Ware, C.F. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov 12,147-168 (2013).
- 12. Ware, C.F., Croft, M. & Neil, G.A. Realigning the LIGHT signaling network to control dysregulated inflammation. J Exp Med 219 (2022).
- 13. Zhang, M., Perrin, L. & Pardo, P. A Randomized Phase 1 Study to Assess the Safety and Pharmacokinetics of the Subcutaneously Injected Anti-LIGHT Antibody, SAR252067. Clin Pharmacol Drug Dev 6,292-301 (2017).
- 14. Perlin, D.S. et al. Levels of the TNF-Related Cytokine LIGHT Increase in Hospitalized COVID-19 Patients with Cytokine Release Syndrome and ARDS. mSphere 5 (2020).
- 15. Perlin, D.S. et al. Randomized, double-blind, controlled trial of human anti-LIGHT monoclonal antibody in COVID-19 acute respiratory distress syndrome. J Clin Invest 132 (2022).
- 16. Sedy, J.R. et al. A herpesvirus entry mediator mutein with selective agonist action for the inhibitory receptor B and T lymphocyte attenuator. J Biol Chem 292,21060-21070 (2017).
 Mar 25, 2025
Mar 25, 2025Engineering antibodies with a novel fusion protein
Mar 25, 2025Fusing two immune system proteins leads to a new method of generating antibodies and may advance drug discovery.
 Nov 19, 2024
Nov 19, 2024Protein superfamily crucial to the immune system experiences Broadway-style revival
Nov 19, 2024San Diego scientists note a resurgence of interest in research on protein family to find new drug candidates.
 Jun 1, 2023
Jun 1, 2023Pumping the brakes on autoimmune disease
Jun 1, 2023New study describes the science behind an autoimmune disease treatment in a Phase 2 clinical trial Researchers at Sanford Burnham…
 Nov 2, 2022
Nov 2, 2022Seeing the immune system in full color
Nov 2, 2022A new flow cytometer at the Institute will help researchers study the immune system with unprecedented resolution and speed. The…
 Apr 6, 2022
Apr 6, 2022How our immune system controls gut microbes
Apr 6, 2022And how this relationship could help fight autoimmune diseases
 Jan 20, 2022
Jan 20, 2022New COVID-19 drug passes phase 2 clinical trial
Jan 20, 2022The new treatment, developed by Avalo Therapeutics with Sanford Burnham Prebys researchers, can mitigate lung damage and improve survival in…
Kristiina Vuori earned her MD and PhD at University of Oulu, Finland. After completion of internship and residency, she received postdoctoral training at the Institute and was appointed to faculty in 1996. Dr. Vuori was selected as a PEW Scholar in the Biomedical Sciences in 1997. She has been co-Director of the Conrad Prebys Center for Chemical Genomics, housed at Sanford Burnham Prebys, since its inception in 2005. She was appointed Deputy Director of the Institute’s NCI-Designated Cancer Center in 2003, and Director of the Cancer Center in 2006. In 2008, she was appointed Executive Vice President for Scientific Affairs at Sanford Burnham Prebys. She was President of the Institute from 2010 to 2022.
Related Disease
Brain Cancer, Breast Cancer, Cancer, Leukemia/Lymphoma, Lung Cancer, Ovarian Cancer, Prostate Cancer
Dr. Vuori’s research is aimed at unraveling the cell mechanisms of the most life-threatening aspect of cancer, which is cancer metastasis. Metastasis is responsible for nearly all deaths in cancer patients, and understanding of the mechanisms that turn a cancer from a locally growing tumor into highly metastatic cancer cells will provide clues how to prevent this step in cancer progression. All cells in our body stick to one another and to the packaging material, or extracellular matrix, around them. This adhesion is essential for cell survival; if cells become detached from their microenvironment, they will die through a process known as apoptosis. This phenomenon, which is called adhesion dependency of survival, is one of the safeguards that maintain the integrity and normal function of tissues, and prevent cells from becoming cancerous. Normal cells cannot detach from their tissue and establish themselves somewhere else, because they will die on the way. Yet cancer cells somehow get around this requirement; they trespass aggressively into other tissues and metastasize to distant sites in the body without dying. Dr. Vuori’s work is aimed at identifying the molecular mechanisms that in normal cells makes them adhesion-dependent; false action of the very same mechanisms is likely to be the key step in allowing cancer cells to metastasize.
 Feb 1, 2024
Feb 1, 2024New genome mapping tool may uncover secrets for treating blood cancers
Feb 1, 2024The outlook for patients with acute myeloid leukemia (AML), a deadly set of blood cancers that is difficult to treat,…
 Nov 14, 2017
Nov 14, 2017Cancer Center Open House Showcases SBP Scientists
Nov 14, 2017SBP’s Cancer Center Open House on November 9, 2017 enlightened visitors from the community on the topic of “The Science…
 Oct 18, 2017
Oct 18, 2017Spectacular 2017 SBP annual Gala celebrates “Sights Set on Discovery”
Oct 18, 2017Friends and supporters of Sanford Burnham Prebys Medical Discovery Institute (SBP) gathered under the stars on Harbor Island in downtown…
 Dec 13, 2016
Dec 13, 2016Reena Horowitz honored at Sanford Burnham Prebys Medical Discovery Institute
Dec 13, 2016During a special end-of-the-year gathering, Reena Horowitz was honored for her hard work and dedication to Sanford Burnham Prebys Medical…
 Jun 8, 2016
Jun 8, 2016SBP selected as designated center for Chemical Biology Consortium
Jun 8, 2016SBP has been chosen as a dedicated center for the Experimental Therapeutics (NExT) Program Chemical Biology Consortium (CBC), centered at…
 Sep 1, 2015
Sep 1, 2015The Mayor of San Diego visits SBP in La Jolla
Sep 1, 2015On Friday, August 28, the Mayor of San Diego, Kevin Faulconer, visited SBP to learn more about how our Institute is…
Charles Spruck earned his BS in Biology at UCLA and PhD in Molecular Biology at the University of Southern California. He worked as a postdoctoral fellow at The Scripps Research Institute in La Jolla and was recruited to the Sidney Kimmel Cancer Center in San Diego as an Assistant Professor in 2003. He joined Sanford Burnham Prebys in 2010.
Education and Training
2003: Post-doc, The Scripps Research Institute
1986: PhD, University of Southern California
1995; BS, University of California at Los Angeles
Prestigious Funding Awards / Major Collaborative Grants
NIH/NCI DoD BCRP CBCRP TRDRP
Honors and Recognition
ACS Scholar
Related Disease
Breast Cancer, Cancer, Lung Cancer, Molecular Biology
Phenomena or Processes
Cancer Biology, Cancer Epigenetics, Cell Biology, Cell Cycle Progression, Cell Signaling, Genomic Instability, Innate Immunity, Metastasis, Posttranslational Modification, Proteolytic Pathways
Research Models
Cultured Cell Lines, Human Cell Lines, Mouse, Mouse Cell Lines
Techniques and Technologies
Cell Biology, Drug Discovery, Gene Knockout (Complete and Conditional), In vivo Modeling
“Despite recent advances in treatment, patients with advanced metastatic cancers have few treatment options. Our lab is focused on developing new effective and non-toxic treatments for these patients.”
Dr. Spruck’s laboratory is focused on developing new, effective, and non-toxic treatments for patients with advanced cancers. The lab focuses on defining the molecular networks that regulate cancer cell division and drive metastasis progression. Recent studies have focused on viral mimicry as a therapeutic approach in cancer, which involves the activation of dormant endogenous retroviruses and retrotransposons in cancer cells to enhance immunogenicity and the effectiveness of immune checkpoint blockade immunotherapy and DNA damaging therapies. The laboratory utilizes various biochemical and molecular approaches, CRISPR gene editing, and animal models of cancer. An emphasis is on studies of breast, lung, prostate, and brain tumors.
Charles Spruck’s Research Report
Developing viral mimicry therapeutic approaches for cancer: Approximately 45% of the human genome is composed of repetitive elements (REs), including endogenous retroviruses and retrotransposons, that are normally transcriptionally silenced in somatic cells. Recent studies suggest that the transcriptional awakening of ERVs/retrotransposons beyond a threshold level of tolerance in cancer cells induces antiviral responses that can enhance the efficacy of certain therapies, including immunotherapy. We recently discovered a novel epigenetic regulatory pathway, FBXO44/SUV39H1, that is essential for ERV/retrotransposon silencing in cancer cells. Preclinical studies showed that FBXO44/SUV39H1 inactivation induces viral mimicry in cancer cells, leading to increased immunogenicity, decreased tumorigenicity, and enhanced the efficacy of immune checkpoint blockade therapy. We are currently exploring therapeutic approaches to target this pathway, and others like it, to prevent tumor growth and enhance immunotherapy response. We are also exploring the role of reactivated REs in human diseases.
Targeting metastatic tumors: Metastasis is a major cause of mortality in cancer. Through genomic screens and biochemical studies, we are identifying novel molecular pathways that drive cancer cell motility, invasion, and metastasis. Recently, we identified a novel molecular axis, FBXO7/EYA2-SCF(FBXW7), that promotes cancer cell motility and cancer stem cell self-renewal and suppresses cancer cell immunogenicity. Targeting this axis prevented metastasis progression, reduced the cancer stem cell population, and stimulated anti-tumor immune responses in preclinical mouse breast cancer models.
 Dec 12, 2025
Dec 12, 2025Sanford Burnham Prebys scientists garner eight cancer research grants from Curebound to advance therapeutic treatments and cures
Dec 12, 2025Ten scientists at Sanford Burnham Prebys Medical Discovery Institute were awarded eight grants yesterday from Curebound, a San Diego-based philanthropic organization
 Apr 24, 2023
Apr 24, 2023Charles Spruck awarded $1.7M to advance “ancient virus” treatment for prostate cancer
Apr 24, 2023The approach uses ancient viruses, embedded in our genomes, to trick the body into thinking it has an infection. With…
 Jun 30, 2022
Jun 30, 2022Three Sanford Burnham Prebys faculty receive promotions
Jun 30, 2022The promoted faculty, all from the Institute’s NCI-designated Cancer Center, include Ani Deshpande, PhD, Brooke Emerling, PhD and Charles Spruck,…
 Mar 15, 2022
Mar 15, 2022Tricking the body to treat breast cancer
Mar 15, 2022With the help of two new grants from the National Institutes of Health totaling more than $4.4 million, Charles Spruck,…
 Nov 3, 2021
Nov 3, 2021Boosting immunotherapy in aggressive brain cancer
Nov 3, 2021Institute have collaborated the University of Pittsburgh Cancer Institute to reveal a new approach to enhance the effects of immunotherapy in…
 Feb 11, 2021
Feb 11, 2021Mining “junk DNA” reveals a new way to kill cancer cells
Feb 11, 2021Scientists unearth a previously unknown vulnerability for cancer and a promising drug candidate that leverages the approach Scientists at Sanford…