Related Disease: Lung Cancer
Kristiina Vuori earned her MD and PhD at University of Oulu, Finland. After completion of internship and residency, she received postdoctoral training at the Institute and was appointed to faculty in 1996. Dr. Vuori was selected as a PEW Scholar in the Biomedical Sciences in 1997. She has been co-Director of the Conrad Prebys Center for Chemical Genomics, housed at Sanford Burnham Prebys, since its inception in 2005. She was appointed Deputy Director of the Institute’s NCI-Designated Cancer Center in 2003, and Director of the Cancer Center in 2006. In 2008, she was appointed Executive Vice President for Scientific Affairs at Sanford Burnham Prebys. She was President of the Institute from 2010 to 2022.
Related Disease
Brain Cancer, Breast Cancer, Cancer, Leukemia/Lymphoma, Lung Cancer, Ovarian Cancer, Prostate Cancer
Dr. Vuori’s research is aimed at unraveling the cell mechanisms of the most life-threatening aspect of cancer, which is cancer metastasis. Metastasis is responsible for nearly all deaths in cancer patients, and understanding of the mechanisms that turn a cancer from a locally growing tumor into highly metastatic cancer cells will provide clues how to prevent this step in cancer progression. All cells in our body stick to one another and to the packaging material, or extracellular matrix, around them. This adhesion is essential for cell survival; if cells become detached from their microenvironment, they will die through a process known as apoptosis. This phenomenon, which is called adhesion dependency of survival, is one of the safeguards that maintain the integrity and normal function of tissues, and prevent cells from becoming cancerous. Normal cells cannot detach from their tissue and establish themselves somewhere else, because they will die on the way. Yet cancer cells somehow get around this requirement; they trespass aggressively into other tissues and metastasize to distant sites in the body without dying. Dr. Vuori’s work is aimed at identifying the molecular mechanisms that in normal cells makes them adhesion-dependent; false action of the very same mechanisms is likely to be the key step in allowing cancer cells to metastasize.
 Feb 1, 2024
Feb 1, 2024New genome mapping tool may uncover secrets for treating blood cancers
Feb 1, 2024The outlook for patients with acute myeloid leukemia (AML), a deadly set of blood cancers that is difficult to treat,…
 Nov 14, 2017
Nov 14, 2017Cancer Center Open House Showcases SBP Scientists
Nov 14, 2017SBP’s Cancer Center Open House on November 9, 2017 enlightened visitors from the community on the topic of “The Science…
 Oct 18, 2017
Oct 18, 2017Spectacular 2017 SBP annual Gala celebrates “Sights Set on Discovery”
Oct 18, 2017Friends and supporters of Sanford Burnham Prebys Medical Discovery Institute (SBP) gathered under the stars on Harbor Island in downtown…
 Dec 13, 2016
Dec 13, 2016Reena Horowitz honored at Sanford Burnham Prebys Medical Discovery Institute
Dec 13, 2016During a special end-of-the-year gathering, Reena Horowitz was honored for her hard work and dedication to Sanford Burnham Prebys Medical…
 Jun 8, 2016
Jun 8, 2016SBP selected as designated center for Chemical Biology Consortium
Jun 8, 2016SBP has been chosen as a dedicated center for the Experimental Therapeutics (NExT) Program Chemical Biology Consortium (CBC), centered at…
 Sep 1, 2015
Sep 1, 2015The Mayor of San Diego visits SBP in La Jolla
Sep 1, 2015On Friday, August 28, the Mayor of San Diego, Kevin Faulconer, visited SBP to learn more about how our Institute is…
Charles Spruck earned his BS in Biology at UCLA and PhD in Molecular Biology at the University of Southern California. He worked as a postdoctoral fellow at The Scripps Research Institute in La Jolla and was recruited to the Sidney Kimmel Cancer Center in San Diego as an Assistant Professor in 2003. He joined Sanford Burnham Prebys in 2010.
Education and Training
2003: Post-doc, The Scripps Research Institute
1986: PhD, University of Southern California
1995; BS, University of California at Los Angeles
Prestigious Funding Awards / Major Collaborative Grants
NIH/NCI DoD BCRP CBCRP TRDRP
Honors and Recognition
ACS Scholar
Related Disease
Breast Cancer, Cancer, Lung Cancer, Molecular Biology
Phenomena or Processes
Cancer Biology, Cancer Epigenetics, Cell Biology, Cell Cycle Progression, Cell Signaling, Genomic Instability, Innate Immunity, Metastasis, Posttranslational Modification, Proteolytic Pathways
Research Models
Cultured Cell Lines, Human Cell Lines, Mouse, Mouse Cell Lines
Techniques and Technologies
Cell Biology, Drug Discovery, Gene Knockout (Complete and Conditional), In vivo Modeling
“Despite recent advances in treatment, patients with advanced metastatic cancers have few treatment options. Our lab is focused on developing new effective and non-toxic treatments for these patients.”
Dr. Spruck’s laboratory is focused on developing new, effective, and non-toxic treatments for patients with advanced cancers. The lab focuses on defining the molecular networks that regulate cancer cell division and drive metastasis progression. Recent studies have focused on viral mimicry as a therapeutic approach in cancer, which involves the activation of dormant endogenous retroviruses and retrotransposons in cancer cells to enhance immunogenicity and the effectiveness of immune checkpoint blockade immunotherapy and DNA damaging therapies. The laboratory utilizes various biochemical and molecular approaches, CRISPR gene editing, and animal models of cancer. An emphasis is on studies of breast, lung, prostate, and brain tumors.
Charles Spruck’s Research Report
Developing viral mimicry therapeutic approaches for cancer: Approximately 45% of the human genome is composed of repetitive elements (REs), including endogenous retroviruses and retrotransposons, that are normally transcriptionally silenced in somatic cells. Recent studies suggest that the transcriptional awakening of ERVs/retrotransposons beyond a threshold level of tolerance in cancer cells induces antiviral responses that can enhance the efficacy of certain therapies, including immunotherapy. We recently discovered a novel epigenetic regulatory pathway, FBXO44/SUV39H1, that is essential for ERV/retrotransposon silencing in cancer cells. Preclinical studies showed that FBXO44/SUV39H1 inactivation induces viral mimicry in cancer cells, leading to increased immunogenicity, decreased tumorigenicity, and enhanced the efficacy of immune checkpoint blockade therapy. We are currently exploring therapeutic approaches to target this pathway, and others like it, to prevent tumor growth and enhance immunotherapy response. We are also exploring the role of reactivated REs in human diseases.
Targeting metastatic tumors: Metastasis is a major cause of mortality in cancer. Through genomic screens and biochemical studies, we are identifying novel molecular pathways that drive cancer cell motility, invasion, and metastasis. Recently, we identified a novel molecular axis, FBXO7/EYA2-SCF(FBXW7), that promotes cancer cell motility and cancer stem cell self-renewal and suppresses cancer cell immunogenicity. Targeting this axis prevented metastasis progression, reduced the cancer stem cell population, and stimulated anti-tumor immune responses in preclinical mouse breast cancer models.
 Dec 12, 2025
Dec 12, 2025Sanford Burnham Prebys scientists garner eight cancer research grants from Curebound to advance therapeutic treatments and cures
Dec 12, 2025Ten scientists at Sanford Burnham Prebys Medical Discovery Institute were awarded eight grants yesterday from Curebound, a San Diego-based philanthropic organization
 Apr 24, 2023
Apr 24, 2023Charles Spruck awarded $1.7M to advance “ancient virus” treatment for prostate cancer
Apr 24, 2023The approach uses ancient viruses, embedded in our genomes, to trick the body into thinking it has an infection. With…
 Jun 30, 2022
Jun 30, 2022Three Sanford Burnham Prebys faculty receive promotions
Jun 30, 2022The promoted faculty, all from the Institute’s NCI-designated Cancer Center, include Ani Deshpande, PhD, Brooke Emerling, PhD and Charles Spruck,…
 Mar 15, 2022
Mar 15, 2022Tricking the body to treat breast cancer
Mar 15, 2022With the help of two new grants from the National Institutes of Health totaling more than $4.4 million, Charles Spruck,…
 Nov 3, 2021
Nov 3, 2021Boosting immunotherapy in aggressive brain cancer
Nov 3, 2021Institute have collaborated the University of Pittsburgh Cancer Institute to reveal a new approach to enhance the effects of immunotherapy in…
 Feb 11, 2021
Feb 11, 2021Mining “junk DNA” reveals a new way to kill cancer cells
Feb 11, 2021Scientists unearth a previously unknown vulnerability for cancer and a promising drug candidate that leverages the approach Scientists at Sanford…
Dr. Commisso obtained his PhD at the University of Toronto in the Department of Molecular Genetics and completed his postdoctoral training at New York University School of Medicine. Dr. Commisso made the seminal discovery that Ras-mutant cancer cells use macropinocytosis as an amino acid supply pathway. His laboratory’s research interests are centered on elucidating the underlying mechanisms of how metabolic stress influences the tumor ecosystem in pancreatic cancer. The lab is particularly interested in identifying metabolic adaptations and dependencies that contribute to tumor progression and can be targeted to develop novel therapeutic modalities for this disease.
Related Disease
Cancer, Colorectal Cancer, Lung Cancer, Pancreatic Cancer
Research in the Commisso lab is focused on biological discoveries that have the potential to lead to novel therapeutic strategies for cancer. Of particular interest to our laboratory are Ras-driven cancers, such as pancreatic cancer, which are extremely aggressive and are in urgent need of new and innovative therapies. The biological process that we study in the lab is called macropinocytosis, a fluid-phase form of bulk endocytic uptake, which we have linked to cancer cell metabolism in Ras-mutated tumors.
Cosimo Commisso’s Research Report
Macropinocytosis is an endocytic mechanism of fluid-phase uptake that produces large intracellular vesicles known as macropinosomes. Macropinosomes are heterogeneous in size and shape and serve to internalize large volumes of extracellular fluid along with the associated membrane. In transformed cells, macropinocytosis is stimulated by oncogenes, such as Ras. Ras proteins are small, membrane-localized GTPases that are activated in response to growth factors and they regulate a variety of outputs, including cell proliferation, survival and invasion. Gain-of-function mutations in Ras-encoding genes cause Ras proteins to be trapped in their active state, leading to the constitutive activation of downstream pathways. The functional consequences of macropinocytosis stimulation in mutant Ras-expressing cells were unknown prior to our work. We have linked macropinocytic uptake in Ras-transformed cells to nutrient delivery and amino acid supply (Commisso et al., 2013). We demonstrated that the inhibition of this nutrient delivery pathway selectively compromises growth of Ras-driven tumors. With the long-term goal of specifically targeting such tumors, we have recently developed stream-lined methodology to detect and grade macropinocytosis in tumor tissue (Commisso et al., 2014). Our work was important for two main reasons. First, cancer cells are dependent on amino acids, such as glutamine, for their growth and survival. Therefore, the targeting of these amino acid supply pathways, such as macropinocytosis, represents a promising strategy in developing anti-cancer therapeutics. Second, macropinocytosis is emerging as a mechanism of entry for a variety of therapeutic agents, such as nanoparticles. Hence, identifying that this uptake pathway is active in Ras-driven tumors may have an impact on how these tumors are treated. The complete understanding of the functional significance of Ras-induced macropinocytosis to carcinogenesis and treatment ultimately depends on having a firm grasp of how this process is regulated and on the ability to specifically control it. To this end, a major research focus of the lab is to advance our understanding of the molecular pathways that drive macropinocytosis, which could lead to the identification of new molecular targets whose inhibition would restrain tumor growth and enhancers that could be manipulated to dial-up the uptake process in drug delivery strategies. Additional research interests in the lab include nutrient sensing pathways that are active in the context of macropinocytic uptake and macropinosome maturation, the process that leads to active protein catabolism within the tumor cell.
 Nov 10, 2025
Nov 10, 2025Factoring in frailty and age to improve pancreatic cancer treatment
Nov 10, 2025Study is first to show how aging allows pancreatic tumors to grow and spread faster, may lead to new personalized…
 Sep 11, 2025
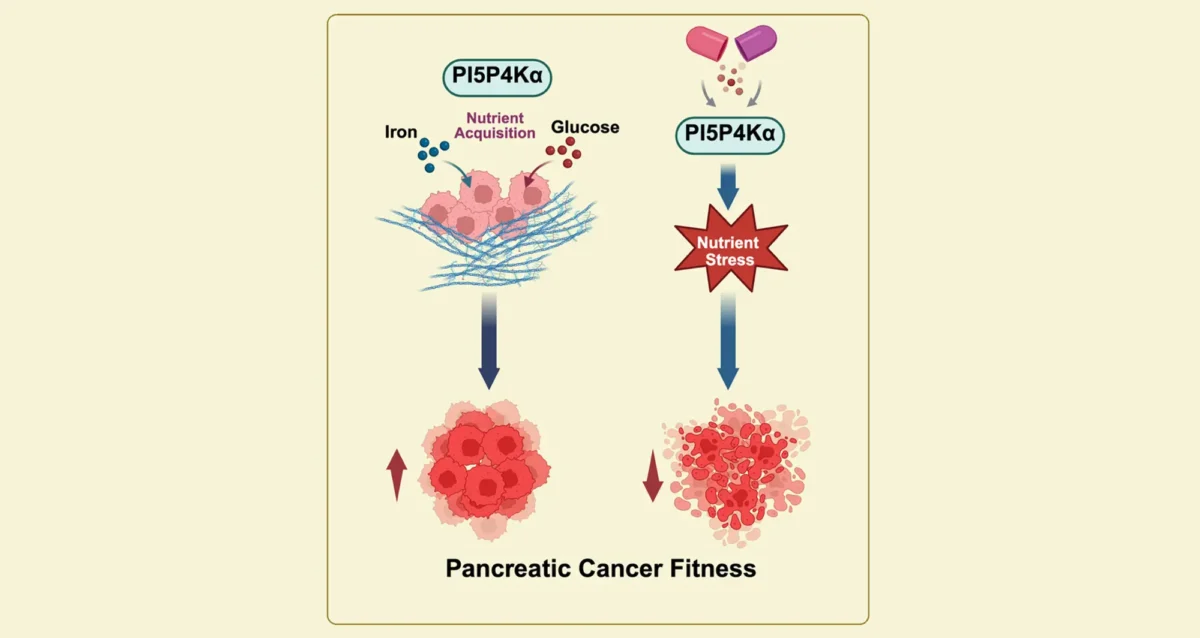
Sep 11, 2025Starving Cancer Cells of Essential Sugar and Iron
Sep 11, 2025Findings from Gurpreet Kaur Arora, Brooke Emerling, and Cosimo Commisso, identify key enzyme that pancreatic cancer cells need to obtain…
 Jul 24, 2025
Jul 24, 2025Reshaping tumor neighborhoods to give treatments a boost
Jul 24, 2025Blocking a fuel source changes tissue near pancreatic tumors, enabling more effective immunotherapy and chemotherapy.
 Dec 5, 2024
Dec 5, 2024Controlling cancer cells’ gluttony for glutamine
Dec 5, 2024Scientists discover new insights about how pancreatic cancer cells adapt when nutrients and other resources are scarce.
 Oct 18, 2024
Oct 18, 2024Two Sanford Burnham Prebys scientists selected for American Cancer Society postdoctoral fellowships
Oct 18, 2024The funds will support Alicia Llorente Lope and Ambroise Manceau who study breast and pancreatic cancer.
 Jul 17, 2024
Jul 17, 2024Sanford Burnham Prebys announces new faculty recruit and two faculty promotions
Jul 17, 2024Douglas Sheffler was named as a new associate professor at Sanford Burnham Prebys, while Cosimo Commisso and Nicholas Cosford garnered…

