The findings could lead to tests or treatments that help prevent type 2 diabetes.
Misfolded proinsulin—a protein the body normally processes into insulin—is an early sign of type 2 diabetes, according to a study by scientists at Sanford Burnham Prebys and the University of Michigan Medical School. The discovery, published in eLife, could lead to tests or treatments that help prevent people from developing type 2 diabetes.
“Understanding the molecular events that occur as prediabetes progresses to diabetes opens new avenues for us to detect or interrupt these processes,” says Randal Kaufman, PhD, director and professor in the Degenerative Diseases Program at Sanford Burnham Prebys and co-corresponding author of the study. “With this information, we can start to find interventions that might spare millions of people from a serious, lifelong condition.”
More than one in three Americans, or approximately 88 million people, have prediabetes—which is characterized by elevated blood sugar. If left untreated, within four years nearly 40% of people with prediabetes develop type 2 diabetes, which occurs when the body doesn’t use insulin properly. In 2017, the cost of treating diabetes exceeded $327 billion, according to the American Diabetes Association. Due to increasing obesity rates, the number of people with the condition—particularly children—is on the rise.
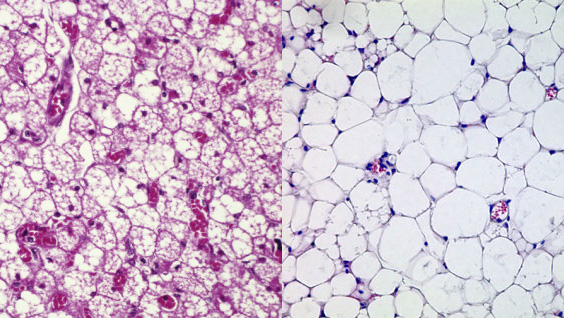
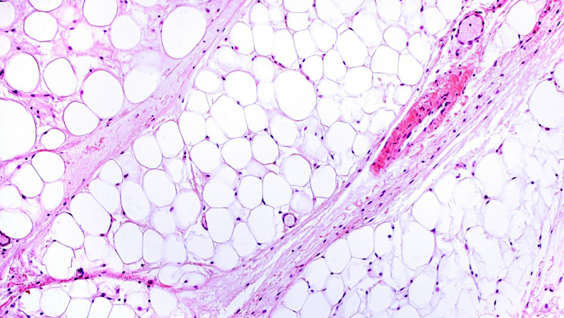
Identifying the molecular events that occur during progression from prediabetes to full-blown diabetes remains one of the most perplexing problems in diabetes research. In the study, the scientists set out to answer this question by tracking proinsulin folding in the beta cells of humans and mice that are healthy, prediabetic and diabetic.
These studies revealed that instead of undergoing its normal folding process, proinsulin proteins were abnormally linked to each other. Levels of the abnormal proinsulin accumulated as prediabetes progressed to type 2 diabetes. Obese mice in the earliest stages of diabetes had the highest levels of abnormal proinsulin in their beta cells.
“Proinsulin misfolding is the earliest known event that may contribute to the progression from prediabetes to diabetes,” says Kaufman. “Together, these studies show that abnormally linked proinsulin holds promise as a potential measure of how close someone may be to developing type 2 diabetes.”
Now, the researchers are set to uncover more details about this process, such as the proteins that interact with the misfolded proinsulin.
“Understanding the fundamental molecular events that lead to type 2 diabetes is critical as the number of people with prediabetes continues to rise,” says Kaufman. “If we don’t find preventive measures, we will soon have a diabetes epidemic.”
The study’s first author is Anoop Arunagiri, PhD; and the study’s senior author is Peter Arvan, both of the University of Michigan Medical School.
Additional authors include Leena Haataja and Fawnnie Pamenan of the University of Michigan Medical School; Ming Liu of the University of Michigan Medical School and Tianjin Medical University in China; Anita Pottekat and Pamela Itkin-Ansari of Sanford Burnham Prebys; Soohyun Kim of Konkuk University in South Korea; Lori M. Zeltser of Columbia University; Adrienne W. Paton and James C. Paton of the University of Adelaide in Australia; and Billy Tsai of the University of Michigan.
The study’s DOI is 10.7554/eLife.44532.
This work was supported by the National Institutes of Health (R01DK111174, R24DK110973 and R01DK48280) and the Juvenile Diabetes Research Foundation International (2-SRA-2018-539-A-B).