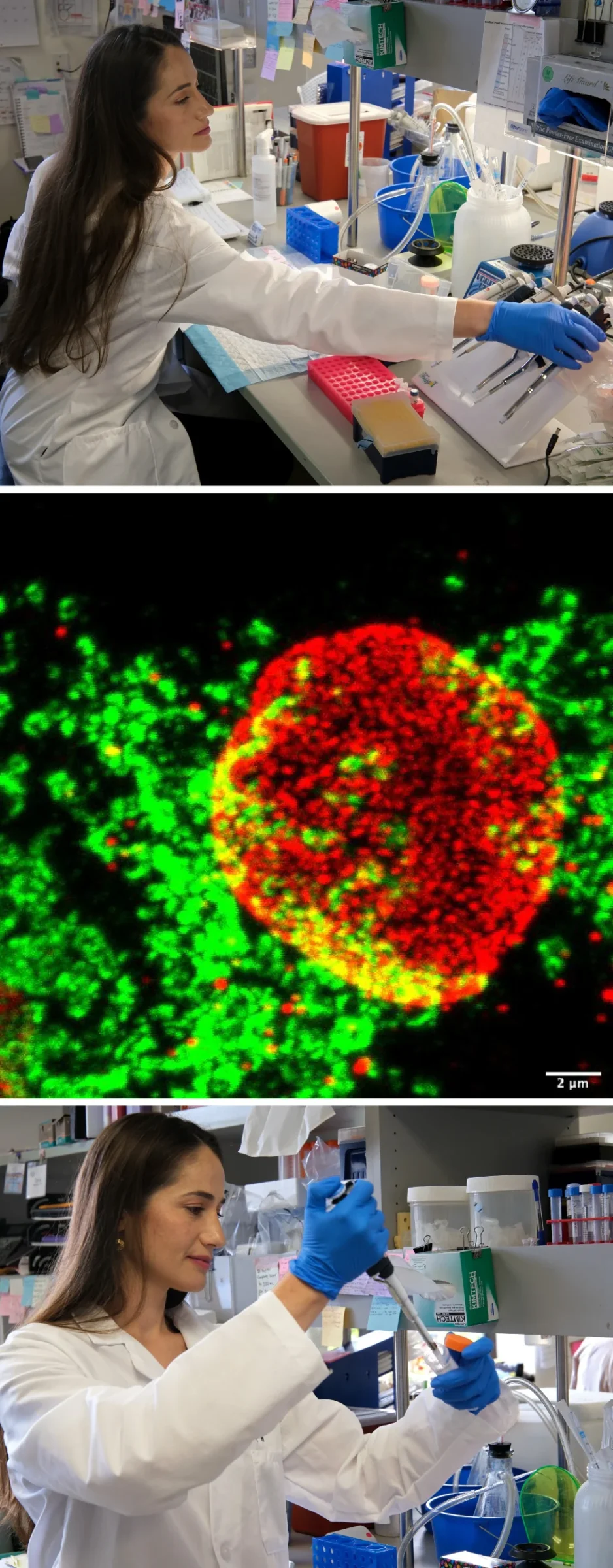
Meet one of our early-career scientists at Sanford Burnham Prebys Medical Discovery Institute: Ambroise Manceau, PhD, a postdoctoral researcher in the lab of Cosimo Commisso, PhD. Manceau studies pancreatic ductal adenocarcinoma — the most common form of pancreatic cancer with only a 13% five-year survival rate.
When and how did you become interested in science?
When I was very young, I was a bit of a nerd when it came to reading reviews of new scientific studies written for kids. Especially anything related to biology.
I lost sight of that interest at some point in my teenage years. I tried going into computer science, but I realized very quickly that it wasn’t a good fit for me. That forced me to do some deeper self-reflection about what I really wanted to do, and that brought me back to biology.
I started studying biology at college, and everything just clicked into place. I really found my way when I went to college.
What brought you to the Commisso lab at Sanford Burnham Prebys?
I have an uncle that I always admired who is a researcher here in California. From talking with him, it sounded like a great place to work and live as a scientist. When I was an undergraduate in France, I decided to do a four-month internship abroad in Los Angeles at the University of Southern California.
I absolutely loved it, and I knew I wanted to return when I could. Because you can finish your graduate school program a bit faster in France, I decided to go back to France to earn my doctorate and then apply for postdoctoral positions in Southern California.
I was looking for labs conducting interesting research in the region, and that is when I found the Commisso lab. It has been a terrific fit for me.
What are the key areas of research you focus on?
My broad focus is on metabolism and organelle biology in pancreatic cancer. My main project looks at macropinocytosis, which is a cellular process that allows cells to gather extra resources from their surrounding environments. Pancreatic cancer cells use this process as an adaptation because they exist in an environment where resources are scarce, and they need to find fuel for their expansion.
I study the contents taken in when pancreatic cancer cells contort their cell surfaces to create pockets called macropinosomes. By analyzing every single protein located on macropinosomes, I found that calcium and zinc transporter proteins present in macropinosomes also are required for macropinocytosis.
These proteins have never been targeted before in pancreatic cancer. By continuing to research them, our long-term goal is to use this strategy to cut the nutrient supply to tumors and see if we can inhibit tumor growth.

What motivates you about your research?
One thing I enjoy is how you adjust your hypothesis based on what you are learning from the experiments. You need to adapt your hypothesis as you gain knowledge, but you don’t always realize it because it can happen one little step at a time.
Then you look at your project a year later, and it is very satisfying to see how much it evolved and how much you changed your mind by following the data.
Also, now that I have attended pancreatic cancer conferences and met with physicians and patients, I have more appreciation for the need to improve upon available therapies.
What do you like about working here?
The people at Sanford Burnham Prebys embrace collaboration. They also are very curious, knowledgeable and kind. With the core facilities, workshops and other opportunities for learning and networking, we have so many resources available to us.
Then add on top of that the location in San Diego, which is a great hub for biomedical research and the biotech industry. And we have the Southern California coast, culture and weather for when we aren’t working.
Have you had an influential mentor?
In addition to my uncle, my thesis mentor and principal investigator back in France were very influential in my professional development. Here, I feel like Cosimo is doing everything he can to get the best out of me, including supporting me to go to workshops and conferences.
What do you enjoy doing when you’re not in the lab?
I’m a bit addicted to rock climbing, and San Diego is a great place to be a climber. I have access to an incredible indoor climbing gym, but I also can go climbing outdoors within a 15-minute drive from where I live.
I also play a bit of tennis, go running and relax at the beach. And I’m painting some, which is something I used to do on rainy days in France. We don’t have many rainy days here, though, so I always want to be outside.
Postdocs at Sanford Burnham Prebys are pushing the boundaries of science every day through curiosity, collaboration, and innovation. This series highlights their unique journeys, what inspires their work, and the impact they’re making across our labs.