Neuroinflammation is the body’s response to injury, infection, toxins, or autoimmune dysfunction in the brain and spinal cord. As in the rest of the body, acute inflammation can help repair harm—primarily by removing damaging elements and dead or dying cells so healing can begin. But when inflammation runs unchecked, it turns destructive.
In neurodegenerative diseases like Alzheimer’s, Parkinson’s, and multiple sclerosis, as well as some psychiatric disorders, chronic or excessive neuroinflammation is a major driver of damage and decline.
Current drug-based treatments for neuroinflammation are limited, in part due tot the blood-brain barrier, which selectively restricts what can pass from the body to the brain, blocking many pharmacological approaches. Existing remedies offer varying degrees of symptom relief for some conditions, but a definitive solution has remained elusive—until now.
In new research published in the January 16, 2026 issue of iScience, part of Cell Press, researchers at Sanford Burnham Prebys Medical Discovery Institute—working with biophysics technology company Fareon and biotechnology company Inapill—describe a new form of magnetic therapy that demonstrated anti-inflammatory, antioxidant, and neuroprotective effects in both in vitro cells and gold-standard animal models of neuroinflammation.
In humans, current magnetic treatments consist primarily of transcranial magnetic stimulation (TMS), which employs high-powered magnetic pulses to stimulate or inhibit nerve cells in the brain, recalibrating neural circuitry to normal function. TMS is most often used in patients with treatment-resistant major depressive disorder.
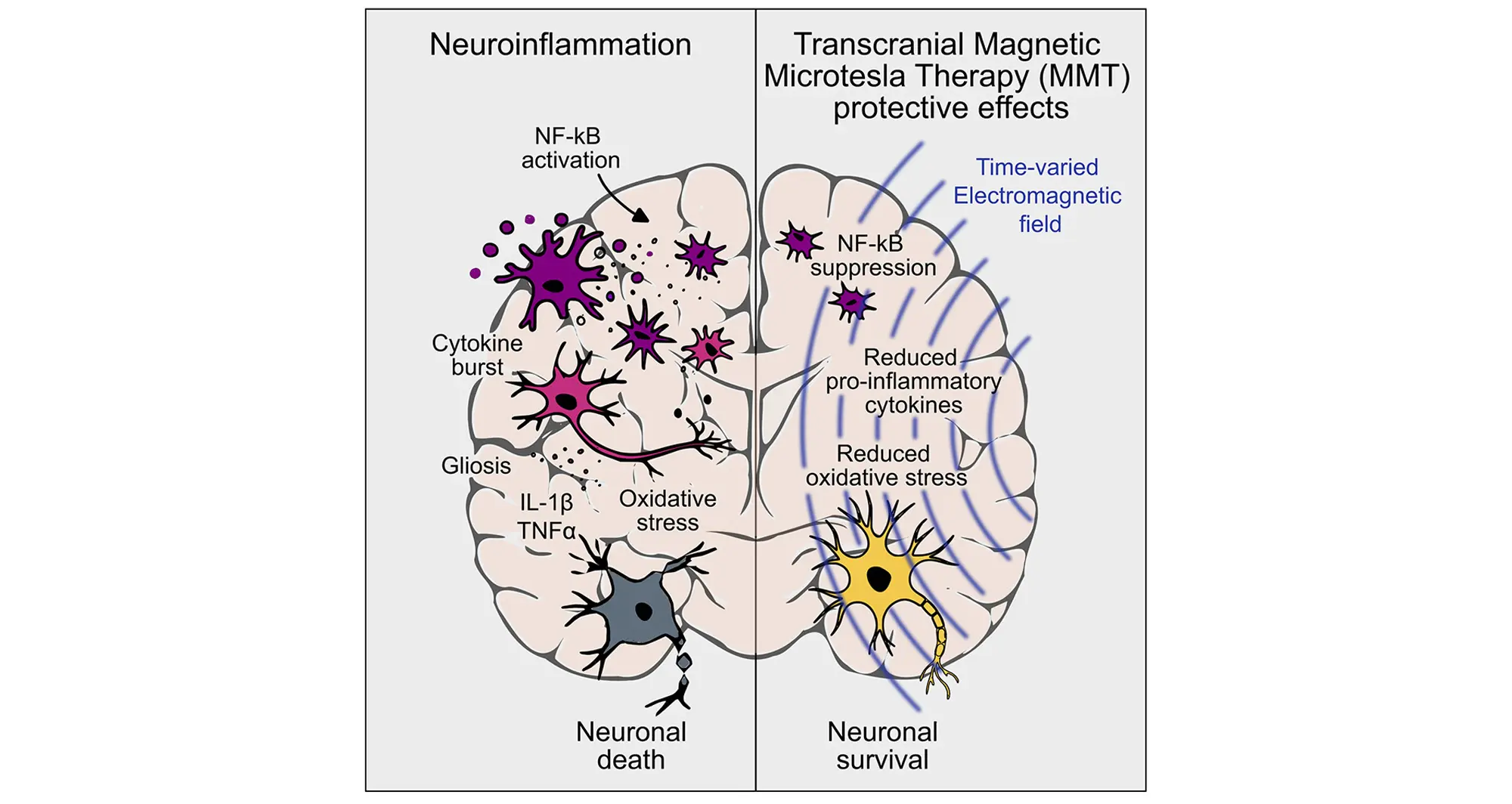
The new research focuses on a novel approach developed by Fareon called microtesla magnetic therapy or MMT. The research team deployed very specific time-varied magnetic fields optimized to inhibit the production of cytokines in human cells and in a rat model of lipopolysaccharide-induced progressive neuroinflammation.
A microtesla is a unit of magnetic field strength. Cytokines are signaling proteins that orchestrate immune responses, including inflammation. Lipopolysaccharide is a bacterial toxin commonly used in research to trigger brain inflammation.
“We found that in cells, MMT inhibited cytokine production and suppressed activation of immune cells like monocytes and macrophages, conferring both indirect and direct neuroprotection,” said study co-author Kevin Tharp, PhD, assistant professor in the Cancer Metabolism and Microenvironment Program at Sanford Burnham Prebys.
“In the animal studies, we were astounded by how well this MMT regimen attenuated the inflammatory properties of microglia and astrocytes. The data were clear: this non-invasive therapy was sufficient to enable regeneration after injury, in part by lessening the magnitude of the inflammatory response.”
The study authors said their findings offer compelling evidence that localized transcranial MMT could become a powerful, noninvasive therapy for neuroinflammatory conditions.
“The rapid anti-inflammatory and neuroprotective effects observed in these studies are very promising and suggest MMT may represent a new class of treatment for unmet needs in neurological diseases, such as Parkinson’s disease, and severe mental health disorders, such as bipolar disorder. We have just completed a first-in-human clinical trial and are excited to share results from that study soon,” said senior author and inventor of the technology Blake Gurfein, PhD, founder and CEO of Fareon.
Additional study authors include:
- Nhu Nguyen and Nathan Brady from Fareon
- Greg Timblin from Inapil, Baker Labs
Disclosure: The authors are employees and/or equity holders of Fareon (formerly Humanity Neurotech), which funded the published research.
The study’s DOI is https://doi.org/10.1016/j.isci.2025.114425.