Findings identify key enzyme that pancreatic cancer cells need to obtain vital metabolic nutrients
Enzymes are an expansive and diverse family of proteins that act as catalysts, typically by binding molecules to speed chemical reactions. They help break down foods, build new molecules and carry out the myriad chemical processes of cells.
They are essential to life, including life mutated by cancer.
“One group of enzymes that cancer cells love to utilize for their rapid proliferation are called phosphoinositide kinases,” said Gurpreet Kaur Arora, PhD, a staff scientist at Sanford Burnham Prebys Medical Discovery Institute.
“They act as messengers and regulators in many essential cellular processes, including those involved in growth and survival. Some of these phosphoinositide kinases are known to support tumorigenesis—the transformation of normal cells into cancer cells and tumors. In particular, phosphatidylinositol 5-phosphate 4-kinases (PI5P4Ks) have garnered attention for their role in cancer metabolism, but their part in the development of pancreatic ductal adenocarcinoma (PDAC), among the most lethal of cancers, was unexplored”.

Gurpreet Kaur Arora, PhD, a staff scientist at Sanford Burnham Prebys.

Brooke Emerling, PhD, director of the Cancer Metabolism and Microenvironment Program.
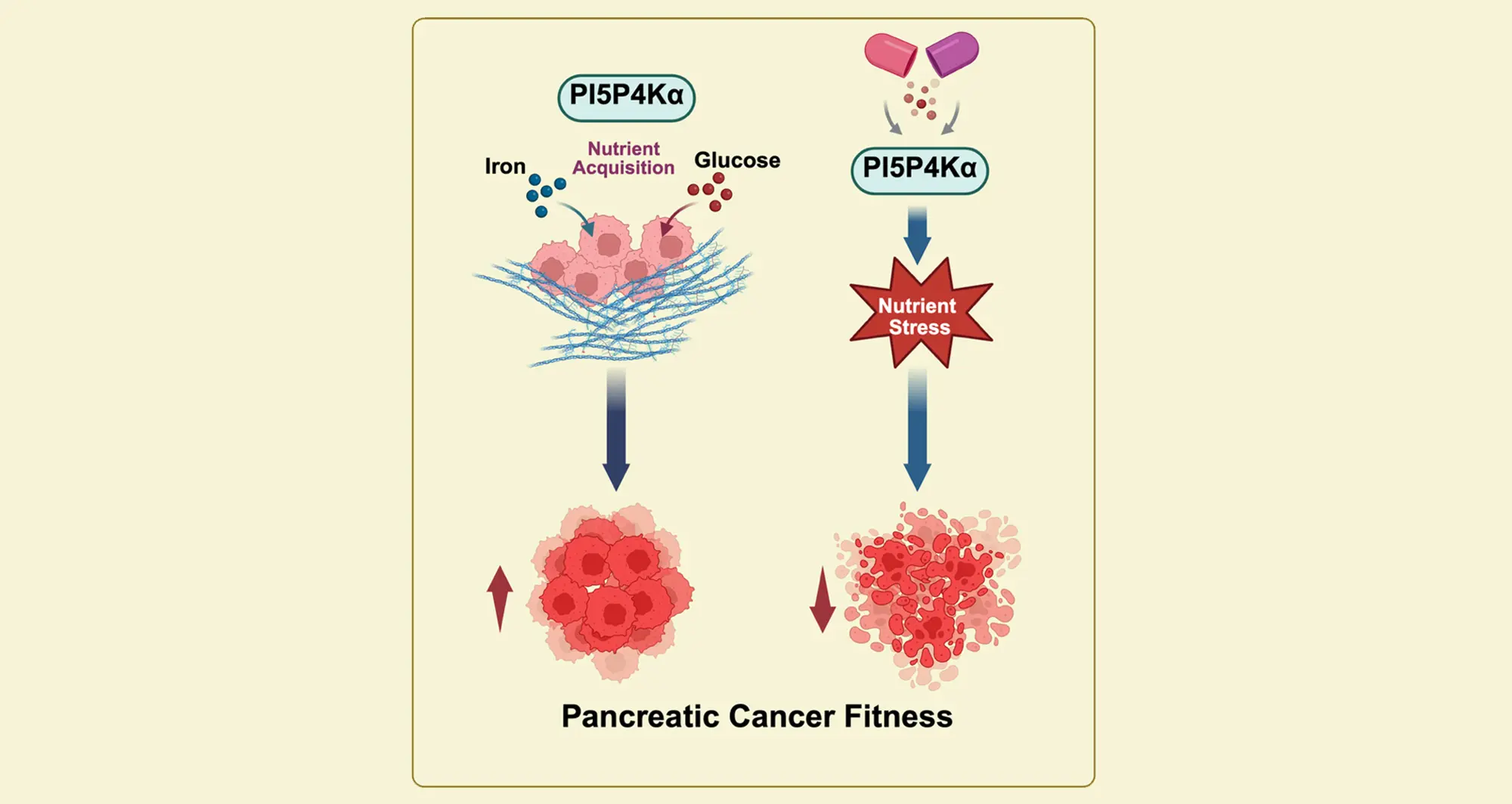
In a new study, published in the journal Cell Reports, a team of scientists at Sanford Burnham Prebys and the University of Zurich in Switzerland, identify a specific phosphoinositide kinase called PI5P4Kα as critical to supporting the unique demands of PDAC cells.
In the research, led by corresponding authors Brooke Emerling, PhD, director of the Cancer Metabolism and Microenvironment Program at Sanford Burnham Prebys, and Cosimo Commisso, PhD, deputy director of the National Cancer Institute-designated cancer center at Sanford Burnham Prebys and the study’s first author Arora, the scientists found that PDAC cells depend upon PI5P4Kα to acquire key ingredients essential to metabolic processes and survival, such as glucose and iron.
And when they induced a knockdown of PI5P4Kα in a xenograft mouse model of PDAC, tumor growth was suppressed.
“Our findings reveal that PI5P4Kα is a critical metabolic vulnerability in p53-mutant pancreatic cancer, where its inhibition disrupts iron homeostasis and glycolytic dependency, leading to cell death,” said Emerling. “Importantly, normal pancreatic cells are spared, underscoring both a mechanistic rationale and a favorable therapeutic window for advancing PI5P4Kα inhibitors in this otherwise treatment-refractory disease.”
“Pancreatic cancer remains one of the deadliest cancers, with far too few effective treatment options for patients,” added Commisso. “Discovering new vulnerabilities is critical, and our work opens the door to a potential new therapeutic strategy. Advances like these are urgently needed to change the trajectory for people facing this devastating disease.”

Cosimo Commisso, PhD, deputy director of the NCI-designated Cancer Center.
Additional study authors include:
Ryan M. Loughran, Kyanh Ly, Cheska Marie Galapate, Alicia Llorente, Taylor R. Anderson, Cynthia Y. Zhang, Shea F. Grenier, Li Ling, Sophia Crabtree, Guillem Lambies, David A. Scott, Yoav Altman, Benji Portillo and Rabi Murad, all at Sanford Burnham Prebys; and Chantal Pauli at the University of Zurich.
The study was supported by funding from the National Cancer Institute, National Institute of General Medical Sciences, the American Cancer Society and the National Institutes of Health.
The study’s DOI is 10.1016/j.celrep.2025.116199.