- Google Scholar Kevin Yip Google Scholar Profile
- H1 Connect F1000 Kevin Yip Faculty Profile
- Bioinformatics micro-modules Watch | Kevin Yip’s Channel for Learning Bioinformatics Concepts, Algorithms and Data Structures
Related Disease: Diabetes - General
Jamey Marth is a Professor at Sanford Burnham Prebys. He has also been Director of the Center for Nanomedicine at the University of California Santa Barbara and Professor in the Department of Molecular, Cellular, and Developmental Biology. Dr. Marth received a PhD degree in Pharmacology from the University of Washington where he trained in the laboratories of Roger M. Perlmutter and Edwin G. Krebs. Dr. Marth’s previous positions included Professor of Medical Genetics at the Biomedical Research Center, University of British Columbia; Professor of Cellular and Molecular Medicine at the University of California San Diego; and Investigator of the Howard Hughes Medical Institute.
Education
1987: PhD, University of Washington, Pharmacology
1984: BS, University of Oregon, Genetics and Chemistry
Honors and Recognition
2017: Karl Meyer Award, Society for Glycobiology
2009-2020: John Carbon Chair in Biochemistry and Molecular Biology
2009-2019: Duncan and Suzanne Mellichamp Chair in Systems Biology
2009: Julius Stone Lectureship Award: Society for Investigative Dermatology
1995-2009: Investigator Award, Howard Hughes Medical Institute
1991-1995: Faculty Scholarship, The Medical Research Council of Canada
Related Disease
Cancer, Colitis, Diabetes – General, Inflammatory/Autoimmune Disease, Sepsis
Dr. Marth is a molecular and cellular biologist specializing in diseases attributable to protein glycosylation. His education and training span molecular genetics, biochemistry, pharmacology, cell biology, immunology, hematology, developmental biology, microbiology, and glycobiology.
As an enzymatic process essential to cells, glycosylation produces saccharides linked by glycosidic bonds to proteins, lipids, and themselves, termed glycans. The vast majority of secreted and cell surface proteins are post-translationally modified by glycosylation during transit through the secretory pathway, termed glycoproteins. A widely used college level cell biology textbook authored by others includes glycans as one of the four main families of the organic molecules of all cells, with lipids, proteins, and nucleic acids and that together they compose the macromolecules and other assemblies of the cell. The structures of glycans (and lipids) are, however, synthesized by template-independent processes, rendering them hard to predict and study. Cells produce and regulate an abundant and diverse glycome of glycosidic linkages in which some of the biological information is decoded by one or more glycan-binding receptors, termed lectins.
Glycans and lectins represent a significant percentage of genes in the genomes of organisms, with several hundred present in mammals. Because glycan biosynthesis, diversification, and degradation rely upon corresponding gene and enzyme function, glycan function can be investigated similarly to other enzymatic and metabolic pathways, such protein phosphorylation. However, we and others found that intact organisms were typically required to discover the functions of protein glycosylation in mammals. His laboratory has focused on discovering the biological information contained within select glycosidic linkages of N- and O-glycans in determining the function and fate of discrete glycoproteins that further contribute to the pathogenesis of autoimmune disease, colitis, diabetes, and sepsis.
To understand the nature and extent of the information generated by glycosidic linkages, we have applied multiple molecular approaches to investigate protein glycosylation in mice and humans. In doing so, we have contributed to the development of enabling technologies with broad applicability, such as conditional mutagenesis by Cre-lox recombination in living animals to determine gene function with temporal and spatial selectivity. His laboratory also develops and studies experimental systems that may better represent real-world models of environmental factors that trigger acquired and common human diseases, results from which have been consistent with clinical findings of human patients. His laboratory includes interdisciplinary team-based collaborations that integrate expertise in immunology, infectious disease, hematology, and more recently, cancer, and is especially focused upon glycosidic linkages attached to the N- and O-glycans of glycoproteins.
The physiological systems regulated by protein glycosylation are broad even when comparing among sequential biosynthetic steps, and our findings continue to indicate the presence of undiscovered information of medical relevance residing in the glycan linkages of glycoproteins.
 May 14, 2025
May 14, 2025Rediscovering the first known cellular receptor
May 14, 2025Scientists from the Marth lab apply new techniques to reexamine a receptor linked to sepsis.
 Oct 31, 2024
Oct 31, 2024Jamey Marth interviewed by The Scientist
Oct 31, 2024The Sanford Burnham Prebys scientist discussed the Cre-loxP recombination system, a mainstay genetic engineering technology.
 Sep 28, 2021
Sep 28, 2021Jamey Marth awarded $13.5 million by NIH to investigate the pathogenesis and treatment of sepsis
Sep 28, 2021Sanford Burnham Prebys professor Jamey Marth, PhD, has been awarded $13.5 million from the National Heart, Lung, and Blood Institute to
 Jul 13, 2021
Jul 13, 2021Study finds promising therapeutic target for colitis
Jul 13, 2021Neu3 controlled the emergence of disease in a model of human colitis An international research group, led by Jamey Marth, PhD,
 Oct 10, 2017
Oct 10, 2017Jamey Marth honored for research linking glycans to diabetes, lupus, sepsis
Oct 10, 2017Jamey Marth, Ph.D., is the 2017 recipient of the Society for Glycobiology’s Karl Meyer Award. The international award is given…
 Jul 26, 2016
Jul 26, 2016Team led by Jamey Marth awarded $12.8M to develop new ways to prevent sepsis
Jul 26, 2016A multidisciplinary team of scientists led by Jamey Marth, PhD, professor in the NCI-designated Cancer Center and director of UC Santa Barbara’s Center
Pamela Itkin-Ansari earned her PhD in Biomedical Sciences from the University of California, San Diego, in 1999. She received postdoctoral training focused on diabetes at that same organization. In 2003, Dr. Itkin-Ansari was appointed Assistant Professor in the Department of Pediatrics, UC San Diego. She moved her laboratory to Sanford Burnham Prebys in 2005.
Funding Awards and Collaborative Grants
California Institute for Regenerative Medicine (CIRM)
Falk Foundation
The Hartwell Foundation
The Hirshberg Foundation
Juvenile Diabetes Research Foundation (JDRF)
National Institutes of Health
Select Honors and Recognition
2013-2014: Outstanding Faculty Mentor Award, UCSD
2011-2014: Hartwell Foundation Biomedical Research Award
2011: Invited Speaker, TEDx
2008-2013: Board of Directors, JDRF San Diego
2008: Health Hero Leadership Award, Combined Health Agencies of San Diego
Other Affiliations
2012-current: Islet Society
2010-current: ASGCT
2008-current: Board of Directors, JDRF San Diego chapter
2008-2013: JDRF board of directors, San Diego
2007-current: American Association for Cancer Research
2007-current: American Diabetes Association
2007-current: American Pediatric Society/Society for Pediatric Research
2006-current: AAAS
Related Disease
Cancer, Diabetes – General, Gastric Cancer, Monogenic Diabetes, Pancreatic Cancer, Type 1 Diabetes, Type 2 Diabetes
Dr. Itkin-Ansari’s research is directed toward understanding diseases of the human pancreas.
Pamela Itkin-Ansari’s Research Report
Diabetes
Areas of focus are: 1) developing a cell-based therapy for diabetes that does not require immunosuppression, and 2) identifying proteins required for proper insulin production and processing.
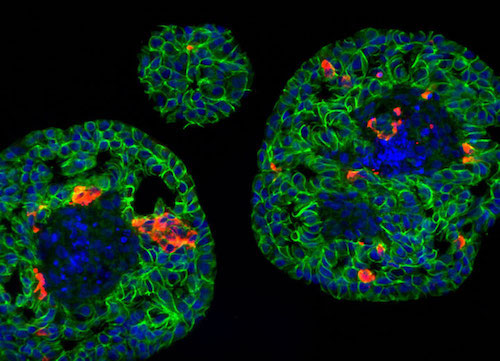
Islet clusters in the developing pancreas
Pancreatic Cancer
The lab determined how dysregulation of specific transcription factors triggers pathogenic cell cycle entry in normal pancreatic cells. This master signaling pathway controlling pancreatic cancer cell growth is yielding potential targets for drug discovery.
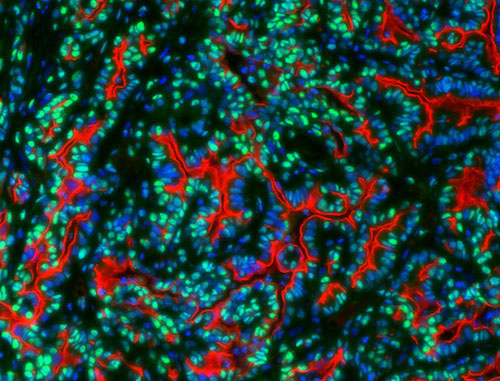
Id3 (green) in human pancreatic cancer
 May 27, 2025
May 27, 2025Scientists and podcasters
May 27, 2025Sanford Burnham Prebys scientists bring dramatic stories of scientific achievement to life.
 May 22, 2024
May 22, 2024Pancreatic cancer symposium celebrates 10th anniversary in San Diego
May 22, 2024The 2024 PancWest Symposium brought more than 120 scientists to the Sanford Burnham Prebys campus in San Diego to discuss…
 Mar 28, 2023
Mar 28, 2023Behind the scenes at Sanford Burnham Prebys’ Cancer Center
Mar 28, 2023Cancer Center open house connects San Diego community with scientists working toward cancer cures
 Oct 26, 2022
Oct 26, 2022Research unlocks the circuitry of diabetes
Oct 26, 2022Research led by Pamela-Itkin-Ansari, PhD, and Randal Kaufman PhD, has mapped out a network of biochemical interactions that help special…
 May 27, 2020
May 27, 2020First map of proinsulin’s “social network” reveals new drug target for type 2 diabetes
May 27, 2020Study reveals previously unknown protein that helps proinsulin fold and opens new avenues for diabetes research Scientists at Sanford Burnham…
 Jan 29, 2016
Jan 29, 2016SBP supports opening of stem cell exhibit at the Reuben H. Fleet Science Center
Jan 29, 2016Pamela Itkin-Ansari, PhD, adjunct assistant professor in the Development, Aging, and Regeneration Program at SBP, participated in the grand opening…