Scientists show how the advanced form of fatty liver disease has monstrous effects on liver cancer risk
Liver cancer has proven to be a tough beast to tame. Experts expected rates of the cancer to decrease following the development of the hepatitis B vaccine in the 1980s, which reduced one of the major risk factors for the disease.
Research in Taiwan showed that its universal infant hepatitis B vaccination program led to young adults experiencing a 35.9% reduction in cases of hepatocellular carcinoma (HCC), the most common liver cancer.
Despite innovation leading to the world’s first cancer-preventing vaccine, incidence of HCC has been on the rise due to a spike in fatty liver disease over recent decades. Lifestyle factors such as high-calorie diets, excessive alcohol consumption and minimal exercise — along with genetic predispositions — can lead to problematic changes in the liver, heart and kidneys.
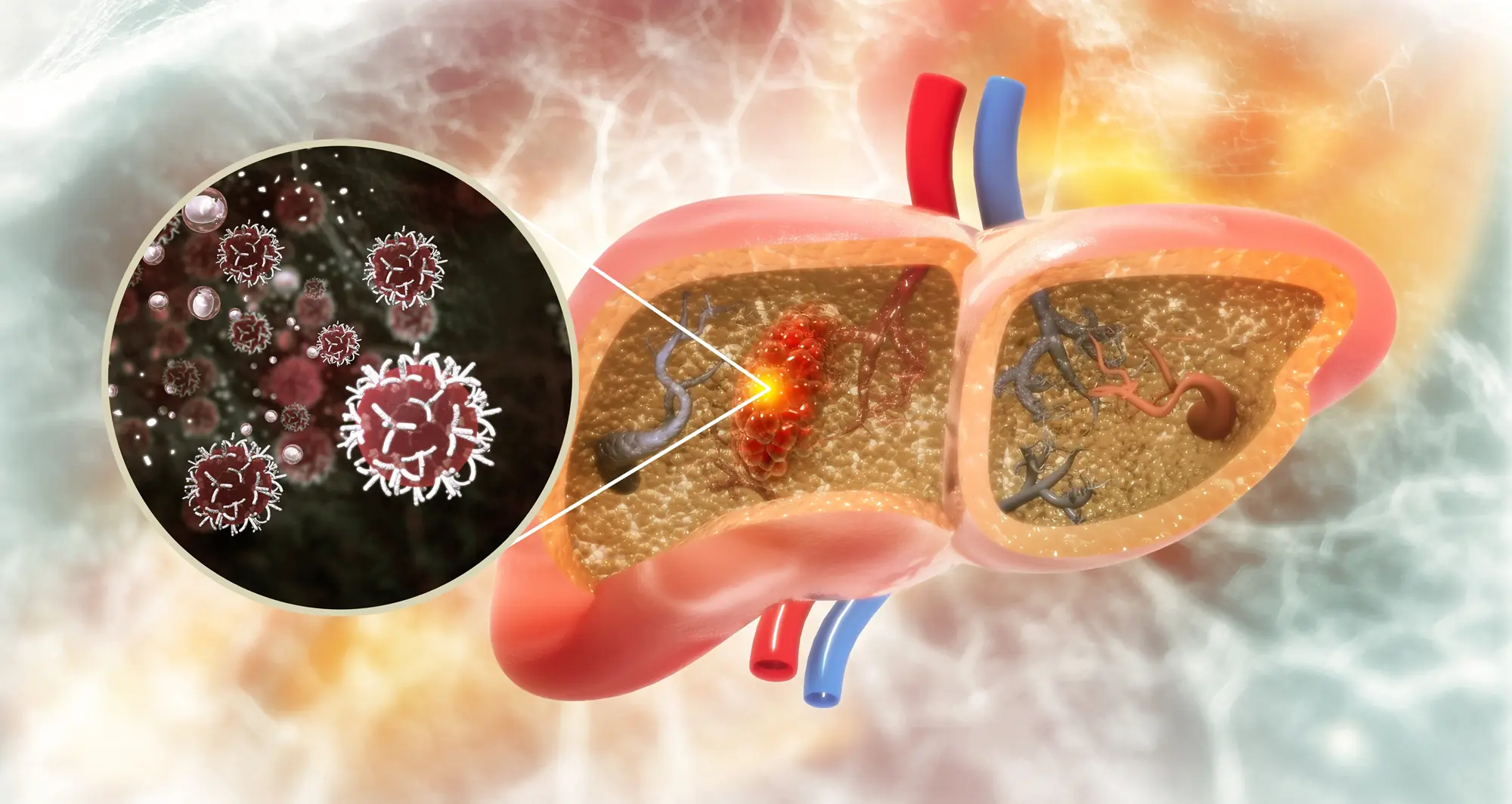
Specifically in the liver, growing deposits of fat in the tissue can lead over time to an advanced form of fatty liver disease marked by chronic inflammation and the accumulation of thickened scar tissue, a condition known as metabolic-associated steatohepatitis (MASH). MASH significantly increases a patient’s risk of developing HCC.

Debanjan Dhar, PhD, is an associate professor in the Cancer Genome and Epigenetics Program.
In a paper published January 1, 2025, in Nature, scientists at Sanford Burnham Prebys, the University of California San Diego, Curtin University, the University of Pennsylvania and The Liver Cancer Collaborative, demonstrated that MASH damages the DNA of liver cells. The study also linked these changes to the development of liver cancer.

Peter Adams, PhD, is the director of the Cancer Genome and Epigenetics Program.
“DNA damage from MASH causes liver cells to stop dividing and enter a zombie-like state called senescence,” said Debanjan Dhar, PhD, associate professor in the Cancer Genome and Epigenetics Program at Sanford Burnham Prebys and coauthor on the study. “This study’s results demonstrate that some of these cells later exit senescence and are likely to become cancerous due to their accumulation of damage and mutations.”
“In the future, we can apply what we’ve learned to study potential opportunities to prevent or repair DNA damage from MASH to reduce patients’ risk of developing liver cancer,” said Peter Adams, PhD, director of the Cancer Genome and Epigenetics Program at Sanford Burnham Prebys and coauthor on the study.
Michael Karin, PhD, Distinguished Professor in the Department of Pharmacology at the University of California San Diego School of Medicine, is the senior and corresponding author on the study.
Li Gu, PhD, a former postdoctoral fellow in the Karin lab, shares first authorship of the study with visiting scientist Yahui Zhu.
Additional authors include:
- Marcos Teneche and Souradipta Ganguly from Sanford Burnham Prebys
- Shuvro Nandi, Maiya Lee, Kosuke Watari, Breanna Bareng, Masafumi Ohira, Yuxiao Liu, Sadatsugu Sakane, Mojgan Hosseini, Tatiana Kisseleva, Ludmil Alexandrov, Consuelo Sauceda and David Gonzalez from the University of California San Diego
- Rodrigo Carlessi and Janina Tirnitz-Parker from Curtin Universit
- The Liver Cancer Collaborative
- M. Celeste Simon from the University of Pennsylvania