Alexander Strongin earned his PhD from Moscow State University in Russia in 1972 and his D.Sci. degree from the Institute of Microbial Genetics in Moscow in 1983. From 1982 to 1988, Dr. Strongin was head of the Laboratory of Functional Enzymology at the Institute of Genetics of Microorganisms in Moscow. He served as head of the Department of Biotechnology and Laboratory of Protein Engineering, Institute of Molecular Genetics, Russian Academy of Sciences, Moscow, from 1988 to 1990. From 1990 to 1994, he was a visiting professor of biochemistry in the Division of Dermatology at Washington University School of Medicine, St. Louis, Missouri. Dr. Strongin has worked in the La Jolla area since 1994, as senior staff scientist in the Biology Division at General Atomics, 1994-1995, and as senior staff scientist at the La Jolla Institute for Experimental Medicine, 1995-1999. Dr. Strongin joined Sanford Burnham Prebys on September 1, 1999.
Related Disease: Arthritis
Yu Yamaguchi earned his MD from Tohoku University in Japan in 1981, followed by a PhD in 1985, and training in obstetrics and gynecology at the same institute. Dr. Yamaguchi came to Sanford Burnham Prebys for his postdoctoral training. He was appointed to the staff in 1991.
Honors and Recognition
The Humanitarian Scientific Achievement Award, The MHE Research Foundation
The Kushima Prize, The Alumni Association, Tohoku University School of Medicine
Other Affiliations
Member, Scientific and Medical Advisory Board, The MHE Research Foundation
Related Disease
Alzheimer’s Disease, Arthritis, Autism Spectrum Disorders, Bone Mineralization Disorders, Epilepsy, Multiple Hereditary Exostoses
The goal of research in the Yamaguchi laboratory is to understand the role of proteoglycans and glycosaminoglycans in the context of development and human disorders. The general strategy is to define the role of proteoglycans and glycosaminoglycans by characterizing the phenotype of mutant mice lacking the synthesis of individual glycosaminoglycans. Specifically, mutant mice lacking the Ext1 and Has genes have been created to study heparan sulfate and hyaluronan, respectively. Recent progress in genetic studies in humans and mice has begun to reveal that deficiencies in glycosaminoglycans can be the causes and/or confounding factors of human childhood disorders. The Yamaguchi lab is now working to clarify the molecular mechanisms of two such disorders (multiple hereditary exostoses and autism) in order to develop new medical treatments.
For more information on the impact of Dr. Yamaguchi’s work, read letters from the patients with MHE.
Yu Yamaguchi’s Research Report
What Are Proteoglycans and Glycosaminoglycans?
Proteoglycans are a family of glycoproteins consisting of a core protein and a various number of long sugar chains called glycosaminoglycans attached to the core protein (Fig. 1). There are four classes of glycosaminoglycans; heparan sulfate, chondroitin sulfate, keratan sulfate, and hyaluronan (hyaluronic acid). Heparin, the anticoagulant widely used in clinics, is a specialized form of heparan sulfate. Although there is ample circumstantial evidence that these glycosaminoglycans have important biological functions, a complete understanding of their function and their relevance to human diseases requires genetic animal models.
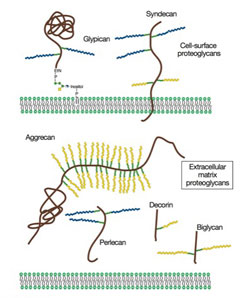
Figure 1. Proteoglycans consist of a protein core and one or more covalently attached glycosaminoglycan chains. From Esko, JD, Kimata, K., and Lindahl, U. Proteoglycans and Sulfated Glycosaminoglycans, In: Essentials of Glycobiology, CSH Press.
Developmental Roles Of Glycosaminoglycans
HEPARAN SULFATE – The Ext1 gene encodes an enzyme essential for the elongation of nascent heparan sulfate chains. As a result of genetic ablation of Ext1 using a ‘conditional knockout’ approach, heparan sulfate is eliminated from specific tissues and cell types. Our previous studies using brain-specific Ext1 conditional knockout have demonstrated critical roles of heparan sulfate in brain patterning, neurogenesis in the cerebral cortex, and pathfinding of various axon tracts (1)(2)(3)(4). More recently, conditional Ext1 knockout in developing limb bones revealed critical roles of heparan sulfate in the growth and patterning of bones and joint formation (5).
Moreover, our conditional Ext1 mutant mouse model has been distributed to more than 20 laboratories worldwide to characterize the role of heparan sulfate in various tissues and cell types, such as colon (6), kidney (7), lymphocytes (8), blood vessels (9), eyes (10), embryonic stem cells (11), and so on.
HYALURONAN – Three Has genes (Has1, Has2, Has3) encode the entire repertoire of hyaluronan synthases in mammalian cells. Genetic ablation of these genes, singly or in combination, results in a reduction or total elimination of hyaluronan, depending on the repertoire of Has expression in the given tissue. We created a conditional null allele of the Has2 gene, which is the predominant Has in many tissues. Our conditional Has2 knockout study targeted to the limb bud mesenchyme has revealed that hyaluronan plays a critical role in the proliferation and maturation of chondrocytes in the developing limb skeleton (12). Like Ext1 mutant mice, these Has2 conditional knockout mice are being used in more than a dozen laboratories worldwide for studies on the role of hyaluronan in various tissue and cell types.
Deficiencies in glycosaminoglycan synthesis can be the causes of childhood disorders
Recent progress in genetic studies in humans and mice has begun to reveal that deficiencies in glycosaminoglycans can be the causes and/or confounding factors of human childhood disorders. The research focus of our lab is to elucidate the molecular mechanisms of such disorders and to develop new medical treatments. We are currently studying two such disorders.
MULTIPLE HEREDITARY EXOSTOSES – One of the major diseases studied in our lab is Multiple Hereditary Exostoses (MHE; also known as Hereditary Multiple Exostoses [HME] or Multiple Osteochondroma [MO]). MHE is caused by a mutation in Ext1 (see above) or its related gene, Ext2. As mentioned above, these genes encode an enzyme necessary to produce heparan sulfate. MHE occurs in children of 0-12 years old. Although no comprehensive survey has been conducted, it is estimated that there are several thousand individuals affected by MHE in the US, which makes MHE one of the more prevalent among ’rare diseases’. Dr. Yamaguchi is a member of the scientific advisory board of the MHE Research Foundation and has been working to promote collaborations between basic scientists, academic physicians, and patient advocates.
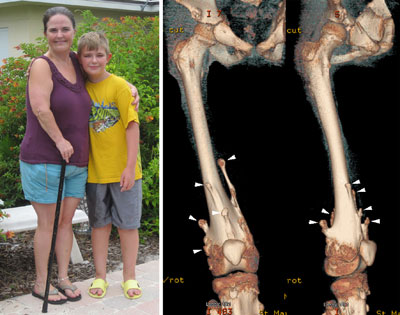
Figure 2. MHE patients, Carol and her 12-year-old son, Bruce. Shown on the right are three-dimensional CT images of Bruce’s right upper leg and knee joint area. Note that there are many bony protrusions (‘exostoses’), as indicated by white arrowheads. These tumors need to be surgically removed to prevent possible malignant transformation. Surgery is also needed to correct bone deformities and bone length inequalities. For example, Bruce and Carol have had 21 and 36 surgeries, respectively.
Children with MHE suffer from the formation of multiple –– sometimes as many as 100 –– bony tumors (osteochondromas) (Fig. 2). These bony tumors stunt their growth and can cause pain and disfigurement. Fortunately, the chance these tumors becomes cancerous is relatively low, partly because they are surgically removed as they develop. This means, however, children with MHE need to go through multiple surgeries over the course of their lives. There is currently no medical treatment for the disease.
Our lab is currently working to elucidate the molecular and cellular mechanisms of MHE. One of the major thrusts has been to create a mouse model that mimics the manifestations of human MHE. A long-term issue of MHE research has been the lack of mouse models that faithfully recapitulate the manifestation of human MHE; when Ext genes were inactivated in mice just as they are in human MHE patients, the mice failed to develop the symptoms of MHE.
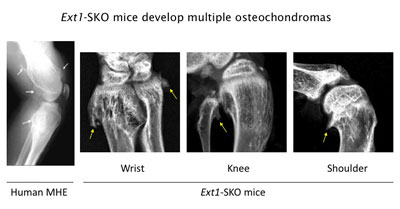
Figure 3. Ext1-SKO mice develop multiple osteochodromas in a pattern almost identical to human MHE. The X-ray images of the knee joint area of an MHE patient and the wrist, knee, and shoulder areas of Ext1-SKO mice. Osteochondromas are indicated by arrows. Ext1-SKO mice also mimic other skeletal deformities frequently seen human MHE, such as bowing of the forearm, the subluxation/dislocation of the radial head, and scoliosis (not shown).
Instead of knocking out the Ext1 gene in the whole mouse, we targeted the gene in only a small fraction of bone cells (Ext1-SKO mice). This minimalistic approach led to a mouse with all the physical manifestations of MHE, such as bony protrusions, short stature, and other skeletal deformities (Fig. 3)(13). The new mouse model answered some long-standing questions about MHE. Scientists had gone back and forth on whether osteochondromas observed in MHE are true tumors or just malformations of the bone. In this study, the tumors were made up of two cell types. A minority were mutant cells lacking Ext1, but, amazingly, most were normal bone cells. Hence, osteochondroma in MHE is not considered a true neoplasm in its strictest sense.
Our lab has been using this and additional mouse models to further dissect the pathogenic mechanism of MHE. Moreover, this mouse model provides new opportunities to test potential drugs to prevent osteochondroma formation and other clinical symptoms of MHE.
AUTISM – Children with MHE sometimes suffer from neurological and mental symptoms, which is not surprising because heparan sulfate is expressed and plays critical roles in the nervous system (1). The MHE community has long noticed the prevalence of autism-like behavioral traits in the patient population, and there are clinical reports describing the association of autism with MHE. Aided by the funds from the Sanford Health and the MHE Research Foundation, we have been studying the behavior of Ext1 mutant mice. Our preliminary data suggest that heparan sulfate has indeed a physiological function in the nervous system, and that its deficiency can cause behavioral deficits relevant to human autism. We are also analyzing DNA samples from individuals with autism for abnormalities in enzymes for heparan sulfate synthesis. Since heparan sulfate is a modulator of a number of neuronal molecules, we hope to identify functional networks of molecules underlying autism and other childhood mental disorders.
 Feb 1, 2022
Feb 1, 2022Sanford Burnham Prebys professor awarded $2.9 million to explore new answers to old questions in Alzheimer’s research
Feb 1, 2022Sanford Burnham Prebys professor Yu Yamaguchi, MD, PhD, has been awarded a $2.9 million grant from the National Institutes of Health
 Oct 21, 2021
Oct 21, 2021This enzyme is one of the hardest working proteins in the body
Oct 21, 2021Researchers have shown that a protein they identified plays a major role in the breakdown of hyaluronic acid, a compound…
 Mar 25, 2019
Mar 25, 2019Families find hope at our 10th Annual Rare Disease Day Symposium
Mar 25, 2019The unofficial theme of Sanford Burnham Prebys’ 10th annual Rare Disease Day symposium can be summarized in one word: hope.
 Jan 9, 2018
Jan 9, 2018Year in review: Top stories in 2017
Jan 9, 2018In the last 12 months, SBP scientists published 338 scientific papers—that’s almost a paper a day. We are proud of…
 Jul 14, 2017
Jul 14, 2017Genes and proteins go hand-in-hand
Jul 14, 2017Thanks to huge improvements in DNA sequencing technology, scientists have identified almost all the genes present in humans. Despite this…
 Mar 15, 2017
Mar 15, 2017Research may explain congenital deafness
Mar 15, 2017If you’ve heard of hyaluronic acid (HA), it’s probably as an ingredient in cosmetic products meant to help keep skin
A career history of fundamental discovery and translational research in immunology has guided Dr. Ware to identify new drug targets and develop novel therapeutics. Dr. Ware’s career in immunology and virology began in 1982 when he became a Professor at the University of California, Riverside’s Division of Biomedical Sciences. In 1996, he joined the La Jolla Institute for Immunology in San Diego as Head of the Division of Molecular Immunology. Professor Ware joined Sanford Burnham Prebys Medical Discovery Institute in 2010, serving as the Director of the Infectious and Inflammatory Disease Center and Adjunct Professor of Biology at the University of California at San Diego. He is currently the Director of the Laboratory of Molecular Immunology, which focuses on discovering and designing immunotherapeutics.
As an educator, he taught medical students immunology and virology. He trained over 60 postdoctoral fellows and graduate students who chose careers in research in academic and pharmaceutical science, patent law, or teaching.
Dr. Ware advises scientific panels and review boards for the National Institutes of Health and serves on the scientific advisor boards for the Allen Institute for Immunology and the Arthritis National Research Foundation. Scientific advisor with several biotechnology and pharmaceutical companies on immunotherapy for cancer and autoimmune diseases using innovative approaches to target discovery and drug development.
Dr. Ware’s research program is dedicated to unraveling the intricate intercellular communication pathways that govern immune responses. His work, which centers on cytokines in the Tumor Necrosis Factor (TNF) Superfamily, particularly those that regulate cell survival and death in response to viral pathogens, spans the domains of cancer,autoimmune and infectious diseases.
At Sanford Burnham Prebys, Dr. Ware is pivotal in promoting the translation of the faculty’s scientific discoveries. His efforts have led to the Institute’s reputation as a productive and preferred partner in collaborations with Pharma, including multi-year research and drug development projects with Eli Lilly and Avalo Therapeutics. His success translating fundamental knowledge into rational drug design has led to three novel therapeutics targeting inflammatory pathways, currently in clinical trials.
Education
1981-1982: T cell Immunology, Dana-Farber Cancer Institute of Harvard Medical School in Boston, MA. Tim Springer and Jack Strominger, advisors.
1979-1981: Biochemistry of Complement, University of Texas Health Science Center, San Antonio, TX. W Kolb, advisor
1974-1979: PhD in Molecular Biology and Biochemistry from the University of California, Irvine; Gale Granger, PhD mentor.
Honors and Recognition
Distinguished Fellow, American Association of Immunologists
Honorary Lifetime Membership Award International Cytokine and Interferon Society
Hans J. Muller-Eberhardt Memorial Lecture
Biotech All Star, San Diego Padres Awar
“Pillars of Immunology” discovery of the Lymphotoxin-b Receptor, published in Science
Outstanding Alumnus, Ayala School of Biological Sciences, University of California, Irvine
National MERIT Award R37 (10 years), National Institute of Allergy and Infectious Disease, NIH
National Research Service Award, NIH Postdoctoral Fellowship
Related Disease
Arthritis, Breast Cancer, Cancer, Crohn’s Disease (Colitis), Infectious Diseases, Inflammatory Bowel Disease, Inflammatory/Autoimmune Disease, Inherited Disorders, Leukemia/Lymphoma, Myeloma, Pathogen Invasion, Psoriasis, Systemic Lupus Erythematosus, Type 1 Diabetes
Research in the Laboratory of Molecular Immunology is directed at defining the intercellular communication pathways controlling immune responses. Our work is focused on the Tumor Necrosis Factor (TNF)-related cytokines in regulating decisions of cell survival and death, especially in responses to viral pathogens. Translational research is redirecting the communication networks of TNF superfamily to alter the course of autoimmune and infectious disease and cancer.
Carl Ware’s Research Report
Discovery of the TNF-LIGHT-LTαβ Network
The molecular elements of this cellular communication network were revealed with our discovery of Lymphotoxin-β and its formation of the trimeric heterocomplex with LTαβ1 and its signaling receptor, Lymphotoxin-β Receptor2. The LTβR revealed a new inter-cellular communication pathway that provides a key mechanism underlying the development and homeostasis of lymphoid organs. A second ligand we discovered, LIGHT (TNFSF14), is a novel ligand for the herpesvirus entry mediator (HVEM; TNFRSF14), and surprisingly, the LTβR3 heralding the concept that TNF, LTαβ, and LIGHT form an integrated signaling network thru distinct receptors controlling inflammation and host defense.4
LTβR Signaling in Host Defense and Immune Evasion
Our investigations into the mechanisms of virus evasion of the immune system revealed an essential role of the LTβR pathway in regulating the type 1 interferon response to cytomegalovirus5 and now recognized as a major defense against other pathogens. LTβR signals differentiate macrophages and stromal cells into IFN-producing cells. LTβR transcriptomics and proteomics datasets we generated revealed a novel constellation of anti-viral host defense mechanisms6. Importantly the role of the LTβR pathway to alter tissue microenvironments by differentiation of specialized stromal cells has implications for promoting effective immune responses to cancer.
Discovery of the HVEM-BTLA Immune Checkpoint
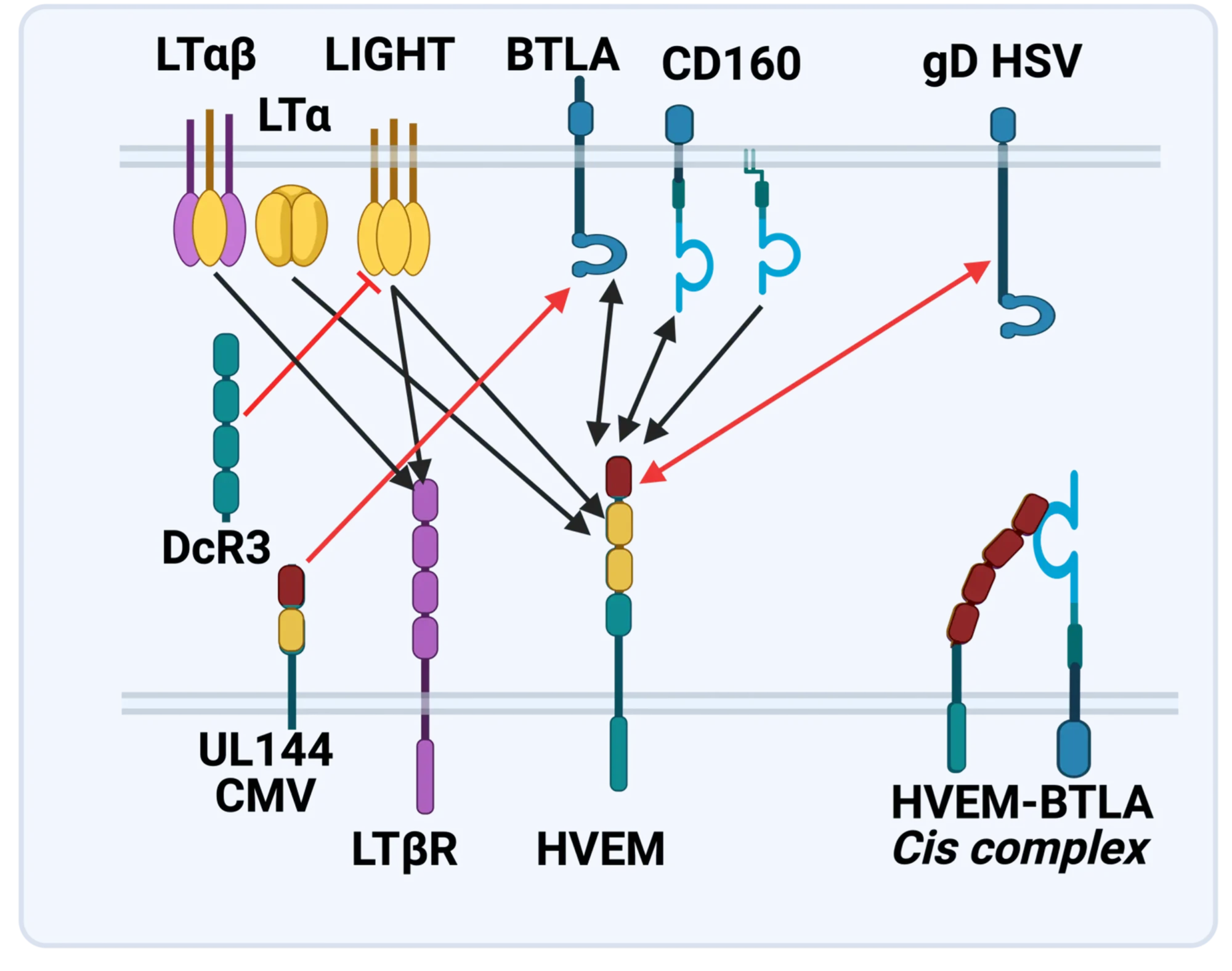
The LTBR-HVEM-BTLA Network in the TNF Superfamilies. Arrows indicate ligand-receptor binding (black), inhibitors (red arrow) and bidirectional signaling (dual arrowheads); HSV, herpes simplex virus; CMV, human cytomegalovirus. BTLA and CD160 are Ig Superfamily proteins. BTLA is an inhibitory checkpoint receptor; DcR3, decoy receptor 3 inhibits LIGHT binding to HVEM and LTBR.
The discovery that HVEM is a coreceptor for the immune checkpoint, B and T lymphocyte attenuator (BTLA), an Ig superfamily member, established a new paradigm in TNF Receptor signaling pathways 7. Additional investigations revealed the importance of the HVEM-BTLA system in limiting immune responses, including T cell help for B cell clonal expansion, antibody maturation, and secretion8. HVEM-BTLA also regulates control of the intestinal microbiome, limiting invasion of pathogenic bacteria and enhancing Treg cell homeostasis 9. The diverse roles of this pathway are seen in the loss of BTLA signaling from mutations in HVEM frequently present in B cell lymphomas10. Additional layers of immune regulators, CD160 and DcR3, control the LIGHT-HVEM-BTLA pathways, revealing this network as a key mechanism controlling immune homeostasis.
Appreciating the fundamental features of the TNF-LIGHT-LTab Network in effector and homeostasis mechanisms presents a target-rich resource for therapeutic intervention in autoimmunity, infection, and cancer11, 12.
Translational research and Immunotherapy
- 2021-current: Lead Scientific Advisor, Avalo Therapeutics
A neutralizing, fully human mAb (quisovalimab) to the proinflammatory cytokine LIGHT (TNFSF14) completed Phase I with an excellent safety profile and a Phase II trial establishing efficacy in COVID-19 pneumonia (NCT0441205)13. We identified elevated serum levels of LIGHT in hospitalized patients with COVID1914 spurring a randomized, double-blind, multicenter, proof-of-concept trial with adults hospitalized with COVID-19-associated pneumonia and mild to moderate ARDS15. The results established efficacy with a significant proportion of patients remaining alive and free of respiratory failure through day 28 after receiving quisovalimab, most pronounced in patients >60 years of age (76.5% vs. 47.1%, respectively; P = 0.042). These results and animal models validated LIGHT as a target for non-COVID inflammatory conditions, clinical trials ongoing in asthma (NCT05288504)12. - 2021-current: Principal Investigator, Avalo Therapeutics – Sanford Burnham Prebys collaboration
Bioengineered a first-in-class checkpoint agonist targeting BTLA immune checkpoint16 in preclinical development - 2019-current: Director and Principal Investigator, Fair Journey Biologics – Sanford Burnham Prebys collaboration
Immunotherapy for TNBC and PANC, in preclinical development - 2015-2022: Director and Lead Principal Investigator, LILLY-Sanford Burnham Prebys Collaboration in Autoimmunity
Collaborative research partnership with Eli Lilly involved target discovery and therapeutic development directed at immune regulators for autoimmune diseases. The collaboration produced three novel biologics currently in Phase I/2 trials (NCT03933943). The collaboration included a target discovery platform for T cell effector memory and NK cell immunomodulators. - 2015-2020: Lead Principal Investigator, Sanford Burnham Prebys – Capella Biosciences collaboration
Created a fully human mAb specific for membrane LIGHT (CBS001); phase I initiated (NCT05323110). - 2016-2020: Lead Scientific Investigator, Boehringer Ingelheim – Sanford Burnham Prebys Collaboration
Target discovery collaboration in inflammatory and fibrotic diseases6 - 2012-2014: Pfizer Innovation Center, Principal Investigator Bioengineering TNFR Superfamily in Autoimmune disease
- 1. Browning, J.L. et al. Lymphotoxin beta, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell 72,847-856 (1993).
- 2. Crowe, P.D. et al. A lymphotoxin-beta-specific receptor. Science 264,707-710 (1994).
- 3. Mauri, D.N. et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity 8,21-30 (1998).
- 4. Ward-Kavanagh, L.K., Lin, W.W., Sedy, J.R. & Ware, C.F. The TNF Receptor Superfamily in Co-stimulating and Co-inhibitory Responses. Immunity 44,1005-1019 (2016).
- 5. Schneider, K. et al. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell Host Microbe 3,67-76 (2008).
- 6. Virgen-Slane, R. et al. Cutting Edge: The RNA-Binding Protein Ewing Sarcoma Is a Novel Modulator of Lymphotoxin beta Receptor Signaling. J Immunol 204,1085-1090 (2020).
- 7. Sedy, J.R. et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol 6,90-98 (2005).
- 8. Mintz, M.A. et al. The HVEM-BTLA Axis Restrains T Cell Help to Germinal Center B Cells and Functions as a Cell-Extrinsic Suppressor in Lymphomagenesis. Immunity 51,310-323 e317 (2019).
- 9. Stienne, C. et al. Btla signaling in conventional and regulatory lymphocytes coordinately tempers humoral immunity in the intestinal mucosa. Cell reports 38,110553 (2022).
- 10. Sedy, J.R. & Ramezani-Rad, P. HVEM network signaling in cancer. Adv Cancer Res 142,145-186 (2019).
- 11. Croft, M., Benedict, C.A. & Ware, C.F. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov 12,147-168 (2013).
- 12. Ware, C.F., Croft, M. & Neil, G.A. Realigning the LIGHT signaling network to control dysregulated inflammation. J Exp Med 219 (2022).
- 13. Zhang, M., Perrin, L. & Pardo, P. A Randomized Phase 1 Study to Assess the Safety and Pharmacokinetics of the Subcutaneously Injected Anti-LIGHT Antibody, SAR252067. Clin Pharmacol Drug Dev 6,292-301 (2017).
- 14. Perlin, D.S. et al. Levels of the TNF-Related Cytokine LIGHT Increase in Hospitalized COVID-19 Patients with Cytokine Release Syndrome and ARDS. mSphere 5 (2020).
- 15. Perlin, D.S. et al. Randomized, double-blind, controlled trial of human anti-LIGHT monoclonal antibody in COVID-19 acute respiratory distress syndrome. J Clin Invest 132 (2022).
- 16. Sedy, J.R. et al. A herpesvirus entry mediator mutein with selective agonist action for the inhibitory receptor B and T lymphocyte attenuator. J Biol Chem 292,21060-21070 (2017).
 Mar 25, 2025
Mar 25, 2025Engineering antibodies with a novel fusion protein
Mar 25, 2025Fusing two immune system proteins leads to a new method of generating antibodies and may advance drug discovery.
 Nov 19, 2024
Nov 19, 2024Protein superfamily crucial to the immune system experiences Broadway-style revival
Nov 19, 2024San Diego scientists note a resurgence of interest in research on protein family to find new drug candidates.
 Jun 1, 2023
Jun 1, 2023Pumping the brakes on autoimmune disease
Jun 1, 2023New study describes the science behind an autoimmune disease treatment in a Phase 2 clinical trial Researchers at Sanford Burnham…
 Nov 2, 2022
Nov 2, 2022Seeing the immune system in full color
Nov 2, 2022A new flow cytometer at the Institute will help researchers study the immune system with unprecedented resolution and speed. The…
 Apr 6, 2022
Apr 6, 2022How our immune system controls gut microbes
Apr 6, 2022And how this relationship could help fight autoimmune diseases
 Jan 20, 2022
Jan 20, 2022New COVID-19 drug passes phase 2 clinical trial
Jan 20, 2022The new treatment, developed by Avalo Therapeutics with Sanford Burnham Prebys researchers, can mitigate lung damage and improve survival in…
Dr. Yu Xin (Will) Wang received his PhD at the University of Ottawa where he identified cellular asymmetry and polarity mechanisms regulating muscle stem cell self-renewal and skeletal muscle regeneration. He then carried out postdoctoral training at Stanford University School of Medicine developing single cell multi-omic approaches to characterize the regenerative process and what goes awry with disease and aging.
“I’ve always had a passion for science and became fascinated with how the body repairs and heals itself when I was introduced to the potential of stem cells in regenerative medicine. I was struck by the ability of a small pool of muscle stem cells that can rebuild and restore the function of muscle. My lab at Sanford Burnham Prebys aims to better understanding the repair process and harness our body’s ability to heal in order to combat chronic diseases and even counteract aging.“
Education and Training
Postdoctoral Fellowship, Stanford University School of Medicine
PhD in Cellular Molecular Medicine, University of Ottawa, Canada
BS in Biomedical Sciences, University of Ottawa, Canada
Prestigious Funding Awards
2020: NINDS K99/R00 Pathway to Independence Award
Honors and Recognition
Governor General’s Gold Medal – Canada
Related Disease
Aging-Related Diseases, Amyotrophic Lateral Sclerosis (Lou Gehrig’s Disease), Arthritis, Cachexia, Inflammatory/Autoimmune Disease, Multiple Sclerosis, Muscular Dystrophy, Myopathy, Neurodegenerative and Neuromuscular Diseases, Sarcopenia/Aging-Related Muscle Atrophy, Spinal Muscular Atrophy
Phenomena or Processes
Adult/Multipotent Stem Cells, Aging, Cell Signaling, Development and Differentiation, Epigenetics, Exercise, Extracellular Matrix, Neurogenesis, Organogenesis, Regenerative Biology, Transcriptional Regulation
Anatomical Systems and Sites
Immune System and Inflammation, Musculoskeletal System, Nervous System
Research Models
Clinical and Transitional Research, Computational Modeling, Human Adult/Somatic Stem Cells, Mouse
Techniques and Technologies
3D Image Analysis, Bioinformatics, Cellular and Molecular Imaging, Gene Knockout (Complete and Conditional), Genomics, High Content Imaging, High-Throughput/Robotic Screening, Live Cell Imaging, Machine Learning, Microscopy and Imaging, Proteomics, Transplantation
The Wang lab is interested in elucidating critical cell-cell interactions that mediate the function of tissue-specific stem cells during regeneration and disease, with a focus on how a coordinated immune response can promote regeneration and how autoimmunity impacts tissue function and hinder repair.
Specifically, the Wang lab aims to identify cellular and molecular crosstalk between muscle, nerve, and immune systems to develop targeted therapies that overcome autoimmune neuromuscular disorders and autoimmune aspects of “inflammaging.”
Yu Xin (Will) Wang’s Research Report
The lab’s research is translationally oriented and utilizes interdisciplinary molecular, genetic, computational (machine learning and neural networks), and bioengineering approaches to view biology and disease from new perspectives. We combine multi-omics sequencing and imaging methods to resolve how different cell types work together after injury to repair tissues and restore function. We use a data-driven approach to identify targetable disease mechanisms and, through collaborations with other researchers and clinicians, develop therapies that promote regeneration. Visit our lab website to learn more.
 Jun 12, 2025
Jun 12, 2025Turning back time on muscle stem cells to prevent frailty from aging
Jun 12, 2025Study from Dr. Will Wang’s lab finds a way to restore stem cells from aged muscle to become young again…
 Aug 20, 2024
Aug 20, 2024Mapping the human body to better treat disease
Aug 20, 2024Scientists are investigating the inner workings of our bodies and the cells within them at an unprecedented level of detail.
 Aug 13, 2024
Aug 13, 2024Dodging AI and other computational biology dangers
Aug 13, 2024Sanford Burnham Prebys scientists say that understanding the potential pitfalls of using artificial intelligence and computational biology techniques in biomedical…
 Oct 11, 2023
Oct 11, 2023Inhibiting an enzyme associated with aging could help damaged nerves regrow and restore strength
Oct 11, 2023New research has demonstrated a way to accelerate recovery from peripheral nerve injury by targeting an enzyme that was thought…
 Jan 26, 2023
Jan 26, 2023Three big questions for cutting-edge biologist Will Wang
Jan 26, 2023Will Wang’s spatial omics approach to studying neuromuscular diseases is unique.
 Nov 23, 2022
Nov 23, 2022Yu Xin (Will) Wang joins Sanford Burnham Prebys to advance regenerative medicine
Nov 23, 2022Molecular biologist Yu Xin (Will) Wang, PhD, has joined Sanford Burnham Prebys as an assistant professor in the Development, Aging,…
Evan Y. Snyder earned his MD and PhD (in neuroscience) from the University of Pennsylvania in 1980 as a member of NIH’s Medical Scientist Training Program (MSTP). He had also studied psychology and linguistics at the University of Oxford. After moving to Boston in 1980, he completed residencies in pediatrics and neurology as well as a clinical fellowship in Neonatal-Perinatal Medicine at Children’s Hospital-Boston, Harvard Medical School. He also served as Chief Resident in Medicine (1984-1985) and Chief Resident in Neurology (1987) at Children’s Hospital-Boston. In 1989, he became an attending physician in the Department of Pediatrics (Division of Newborn Medicine) and Department of Neurology at Children’s Hospital-Boston, Harvard Medical School. From 1985-1991, concurrent with his clinical activities, he conducted postdoctoral research as a fellow in the Department of Genetics, Harvard Medical School. In 1992, Dr. Snyder was appointed an instructor in neurology (neonatology) at Harvard Medical School and was promoted to assistant professor in 1996. He maintained lab spaces in both Children’s Hospital-Boston and at Harvard Institutes of Medicine/Beth-Israel Deaconess Medical Center. In 2003, Dr. Snyder was recruited to Sanford Burnham Prebys as Professor and Director of the Program in Stem Cell and Regenerative Biology. He then inaugurated the Stem Cell Research Center (serving as its founding director) and initiated the Southern California Stem Cell Consortium. Dr. Snyder is a Fellow of the American Academy of Pediatrics (FAAP). He also received training in Philosophy and Linguistics at Oxford University.
Related Disease
Alzheimer’s Disease, Amyotrophic Lateral Sclerosis (Lou Gehrig’s Disease), Arthritis, Brain Cancer, Brain Injury, Breast Cancer, Cancer, Childhood Diseases, Congenital Disorders of Glycosylation, HIV-Associated Dementia, Huntington’s Disease, Multiple Sclerosis, Muscular Dystrophy, Neurodegenerative and Neuromuscular Diseases, Neurological and Psychiatric Disorders, Parkinson’s Disease, Peripheral Vascular Disease, Skin Cancer and Melanoma, Spinal Cord Injury, Stroke, Traumatic Injury
We believe the study of stem cell biology will provide insights into many areas: developmental biology, homeostasis in the normal adult, and recovery from injury. Indeed, past and current research has already produced data in these areas that would have been difficult or impossible via any other vehicle. We have engaged in a multidisciplinary approach, simultaneously exploring the basic biology of stem cells, their role throughout the lifetime of an individual, as well as their therapeutic potential. Taken together, these bodies of knowledge will glean the greatest benefit for scientists and, most importantly, for patients. All of our research to date has been preformed in animal models with the ultimate goal of bringing them to clinical trials as soon as possible. Stem cells offer an intriguing mix of controversy, discovery, and hope. Politicians are charged with dealing with the controversial facets of stem cells, as we prefer to focus our energy on their potential for discovery and hope.
The Snyder Lab studies stem cell biology, with the goal of understanding normal development, tissue homeostasis, and recovery from injury and disease. A major focus is neural stem cells (NSCs), which can self-renew and differentiate into neurons, astrocytes, and oligodendrocytes. These properties make NSCs ideal for repair of damage due to injury or disease, but they also make them susceptible to transformation into malignant cancers.
 May 28, 2025
May 28, 2025Evan Snyder named Fellow of the Child Neurology Society
May 28, 2025Evan Snyder, MD, PhD, has been named a Fellow of the Child Neurology Society, honoring a distinguished career and…
 Nov 11, 2024
Nov 11, 2024Seven questions for FDA advisor Evan Snyder
Nov 11, 2024Sanford Burnham Prebys physician-scientist advises the FDA on cell-based therapeutics, tissue engineering and gene therapies.
 Jul 23, 2024
Jul 23, 2024Mini lungs make major COVID-19 discoveries possible
Jul 23, 2024Scientists infect miniature lung organoids with the virus responsible for COVID-19, revealing new ways in which the infection spreads and…
 Jan 25, 2022
Jan 25, 2022New CIRM grant to fund research internships for underrepresented high school students
Jan 25, 2022Thanks to a new grant awarded to Sanford Burnham Prebys by the California Institute for Regenerative Medicine (CIRM), 57 California…
 Nov 18, 2021
Nov 18, 2021From child neurology to stem cells: An interview with Evan Snyder
Nov 18, 2021What do Evan Snyder and Sigmund Freud have in common? Both radically changed how we see the human brain. In…
 Jul 26, 2021
Jul 26, 2021Biomarker could help diagnosis schizophrenia at an early age
Jul 26, 2021Research could lead to blood-based diagnostic test Scientists at Sanford Burnham Prebys have discovered how levels of a protein could…
After receiving his early training in clinical chemistry/biochemistry at the University of Buenos Aires, Argentina, Dr. Millán first joined the La Jolla Cancer Research Foundation (LJCRF) in 1977, the predecessor of Sanford Burnham Prebys, as a trainee in clinical enzymology. He completed his PhD studies in Medical Biochemistry at the University of Umeå, Sweden and after post-doctoral stints in Copenhagen and LJCRF he was appointed to the faculty at SBP in 1986. He served as Professor of Medical Genetics in the Department of Medical Biosciences at his alma mater, Umeå University, Sweden, from 1995-2000. He was appointed Sanford Investigator at the Sanford Children’s Health Research Center at Sanford Burnham Prebys in 2008.
Honors and Recognition
2018: ASBMR Lawrence G. Raisz Award for Pre-clinical Research.
2001: Gold Medal of the Royal Academy of Medicine and Surgery, Murcia, Spain
1992: Honorary title of AcadémicoCorresponsal at the Royal Academy of Medicine and Surgery, Murcia, Spain.
Other Affiliations
Member, Scientific Advisory Board, Soft Bones
Related Disease
Arthritis, Bone Mineralization Disorders, Cardiovascular Diseases, Colorectal Cancer, Crohn’s Disease (Colitis), Heart Disease, Inherited Disorders, Metabolic Syndrome, Peripheral Vascular Disease, Testicular Cancer
Phenomena or Processes
Cardiovascular Biology, Disease Therapies, Extracellular Matrix, Protein Structure-Function Relationships
Anatomical Systems and Sites
Cardiovascular System, Musculoskeletal System, Vasculature
Research Models
Mouse
The Millán laboratory works on understanding the mechanisms that control normal skeletal and dental mineralization and elucidating the pathophysiological abnormalities that lead to heritable soft bones conditions such as Hypophosphatasia (HPP) and to soft-tissue calcification, including vascular calcification, that is a hallmark in patients affected by a variety of rare genetic diseases as well as in chronic kidney disease. Dr. Millán’s research has already contributed to the implementation of a novel therapy for HPP, a genetic disease caused by deficiency in tissue-nonspecific alkaline phosphatase (TNAP) function, that leads to accumulation in the extracellular space of inorganic pyrophosphate (PPi), a potent inhibitor of mineralization. HPP is characterized by defective mineralization of bones (rickets or osteomalacia), and teeth that display a lack of acellular cementum, hypomineralized dentin and enamel, and periodontal defects. Dr. Millán’s team has demonstrated the effectiveness of enzyme replacement therapy using mineral-targeted recombinant TNAP (asfotase alfa) to prevent the skeletal and dental defects in the TNAP knockout mouse model of infantile HPP. This therapy was approved in 2015 for the treatment of patients with pediatric-onset HPP.
Current efforts, in collaboration with Professor Miyake’s group in Japan (https://www.nms-gt.org/en/members), focus on developing gene therapy as an alternative approach to treat HPP. Dr. Millán’s group has also identified key pathophysiological changes that lead to calcification of the arteries in animal models of generalized arterial calcification of infancy, pseudoxanthoma elasticum and related genetic diseases as well as in animal models of chronic kidney disease. His group, in collaboration with scientists at the Conrad Prebys Center for Chemical Genomics at Sanford Burnham Prebys, has developed proprietary compounds able to ameliorate the soft-tissue calcification in these conditions and clinical trials are now underway using these first-in-class small molecule inhibitors.
 Feb 3, 2025
Feb 3, 2025Gene therapy may be “one shot stop” for rare bone disease
Feb 3, 2025New study shows a single shot may be able to treat a skeletal disease currently requiring nearly daily injections.
 Jul 21, 2023
Jul 21, 2023José Luis Millán joins international initiative to study calcification in aging
Jul 21, 2023José Luis Millán, PhD, has joined a five-year, $13 million program that will study misplaced calcification in the eyes and…
 May 10, 2023
May 10, 2023Where science meets patients: Sanford Children’s Research Center hosts inaugural symposium
May 10, 2023The event celebrated 16 years of progress at the Center and connected scientists with the people most impacted by their work.
 Dec 19, 2022
Dec 19, 2022Sanford Burnham Prebys announces start of Phase 2 clinical trial of DS-1211 in individuals with PseudoXanthoma Elasticum
Dec 19, 2022San Diego Biotech Networks News
 Nov 30, 2022
Nov 30, 2022Sanford Burnham Prebys announces start of Phase 2 clinical trial of DS-1211 in individuals with pseudoxanthoma elasticum
Nov 30, 2022A Phase 2 clinical trial has started of DS-1211 in individuals with pseudoxanthoma elasticum (PXE), a rare multisystem genetic disease…
 Jun 7, 2021
Jun 7, 2021Study supports gene therapy as a promising treatment for soft bone disease
Jun 7, 2021A single dose of AAV8-TNAP-D10 may be safe and effective for hypophosphatasia A preclinical study led by scientists at Sanford