Alexander Strongin earned his PhD from Moscow State University in Russia in 1972 and his D.Sci. degree from the Institute of Microbial Genetics in Moscow in 1983. From 1982 to 1988, Dr. Strongin was head of the Laboratory of Functional Enzymology at the Institute of Genetics of Microorganisms in Moscow. He served as head of the Department of Biotechnology and Laboratory of Protein Engineering, Institute of Molecular Genetics, Russian Academy of Sciences, Moscow, from 1988 to 1990. From 1990 to 1994, he was a visiting professor of biochemistry in the Division of Dermatology at Washington University School of Medicine, St. Louis, Missouri. Dr. Strongin has worked in the La Jolla area since 1994, as senior staff scientist in the Biology Division at General Atomics, 1994-1995, and as senior staff scientist at the La Jolla Institute for Experimental Medicine, 1995-1999. Dr. Strongin joined Sanford Burnham Prebys on September 1, 1999.
Related Disease: Brain Cancer
William Stallcup earned his PhD in biochemistry from the University of California at Berkeley in 1972. He did postdoctoral work at the Salk Institute, where he was appointed Assistant Professor in 1976. Dr. Stallcup was recruited to Sanford Burnham Prebys in 1984.
Related Disease
Atherosclerosis, Brain Cancer, Breast Cancer, Multiple Sclerosis, Obesity, Skin Cancer and Melanoma
Molecules that control cell proliferation and motility are very important for both normal development and the pathology of many diseases. During development, immature precursor cells must undergo an extensive program of cell division in order to generate the millions of cells required to form a mature organism. In many cases these cells must also be able to migrate long distances to reach their final resting spots in the body. In diseases such as cancer, the mechanisms regulating both cell proliferation and migration are disturbed so that cells divide and migrate in an uncontrolled manner. Dr. Stallcup’s laboratory studies a cell surface protein called NG2 that appears to be involved in both cell proliferation and motility. During normal development, NG2 is found on immature cells that are actively dividing and migrating in tissues such as the brain and vasculature. When cells in these tissues mature, they no longer produce NG2. However, when cells become injured or cancerous, they once again produce NG2 and re-acquire the ability to divide and migrate. Work in the Stallcup lab is aimed at understanding the regulation of NG2 so that cell proliferation and motility can be controlled in pathological situations.
William Stallcup’s Research Report
Cell Surface Molecules in the Developing Nervous System
The NG2 chondroitin sulfate proteoglycan is a membrane-spanning protein expressed by several types of immature progenitor cells, including oligodendrocyte progenitors, chondroblasts, skeletal muscle myoblasts, smooth muscle cells, and pericytes. NG2 is also expressed by several types of highly malignant neoplasms, including melanomas, glioblastomas, and lymphomas (Burg et al, 1998). Both the progenitor cells and the tumor cells are mitotic and in some cases highly motile. There is evidence to suggest that NG2 plays a role in both growth control and motility during the development of these cell types. We are currently investigating systems in which NG2 appears to be involved in the signaling mechanisms that control these processes. We have identified three classes of signal transduction mechanisms that may be mediated or modulated by NG2.
NG2 is required for optimal activation of the PDGF alpha receptor by PDGF-AA. NG2-positive smooth muscle cells migrate and proliferate well in response to both PDGF-AA and PDGF-BB, while NG2-negative smooth muscle cells (derived from NG2 knockout mice) respond only to PDGF-BB (Grako et al, 1999). Since we can show that the alpha receptor does not undergo autophosphorylation in response to PDGF-AA, we believe the defect in the NG2-negative cells must lie at the level of receptor activation. We have now demonstrated that NG2 is capable of binding PDGF-AA (but not PDGF-BB) with fairly high affinity, and thus may participate in sequestering the growth factor or in presenting it to the signaling receptor (Goretzki et al, 1999). In this case, therefore, we believe that NG2 plays an auxilliary role to the actual signal transducing molecule.
NG2-positive smooth muscle cells and several NG2-positive cell lines also proliferate and migrate well in response to soluble type VI collagen, an extracellular matrix ligand for the proteoglycan. In contrast, the NG2-negative counterparts of these cells respond much less effectively. We have preliminary indications that the response to soluble type VI collagen involves activation of the MAP kinases Erk-1 and 2, similar to what is seen during stimulation with growth factors. Further experiments will be required to elucidate the details of this signaling cascade and to determine whether NG2 is involved directly or indirectly in activating this pathway.
The third signaling mechanism is observed upon engagement of NG2 by the substratum, a process that results in cell spreading and migration. Importantly, spreading and migration do not occur with cells expressing NG2 variants lacking the cytoplasmic domain of the proteoglycan, suggesting that interaction of NG2 with cytoplasmic ligands is required for these processes to take place. Analysis of the cytoskeletal rearrangements taking place in spreading cells reveals the extension of both filopodia and lamellipodia in response to NG2 engagement (Figure 1). These two processes are thought to be controlled by activation of the rho family GTPases cdc42 and rac, respectively, suggesting that NG2 engagement triggers activation of these GTPases. We are currently investigating the details of the signaling cascades involved in these phenomena, as well as attempting to identify cytoplasmic binding partners for NG2 which may serve as effector molecules for activation of downstream signaling events.
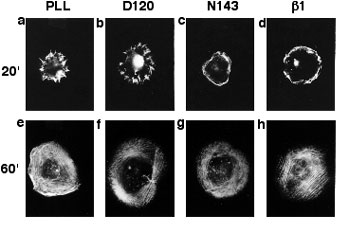
Organization of Actin in Spreading U251 Transfectants
Wild type NG2 transfectants were allowed to spread for 20 (a-d) or 60 (e-f) minutes on surfaces coated with PLL, mAb D120, mAb N143, or mAb beta(1). Cells were then fixed with two percent paraformaldehyde and stained with rhodamine phalloidin to allow visualization of filamentous actin. Radial actin spikes characteristic of filopodia were seen on PLL and mAb D120, while cortical actin bundles characteristic of membrane ruffles were seen on mAb N143 and mAb beta(1). After 60 minutes, stress fibers were apparent in all cases.
Erkki Ruoslahti earned his MD and PhD from the University of Helsinki in Finland in 1967. After postdoctoral training at the California Institute of Technology, he held various academic appointments with the University of Helsinki and the University of Turku in Finland and City of Hope National Medical Center in Duarte, California. He joined Sanford Burnham Prebys in 1979 and served as its President from 1989-2002. He was a Distinguished Professor at University of California Santa Barbara in Biological Sciences 2005-2015. His honors include elected membership to the U.S. National Academy of Sciences, National Academy of Medicine, American Academy of Arts and Sciences, and the European Molecular Biology Organization, the Japan Prize, Gairdner Foundation International Award, G.H.A. Clowes Award, Robert J. and Claire Pasarow Foundation Award, and Jacobaeus International Prize. He was a Nobel Fellow at the Karolinska Institute in Stockholm in 1995, and is an Honorary Doctor of Medicine from the University of Lund, as well as a Knight and Commander of the Orders of the White Rose the the Lion of Finland. In 2022, Dr. Ruoslahti was announced as one of three winners of the Albert Lasker Basic Medical Research Award.
Education
1966: MD, University of Helsinki in Finland
1967: PhD, University of Helsinki in Finland
Awards and Honors
2022: Albert Lasker Award for Basic Medical Research
Commander of the Order of the Lion of Finland
Knight of the Order of the White Rose of Finland
2012: Thomson Reuters Citation Laureate
2005: Japan Prize in Cell Biology
2003: Jubilee Lecturer, Biochemical Society
1998: Jacobaeus International Prize
1997: Gairdner Foundation International Award
1995: Nobel Fellow at the Karolinska Institutet in Stockholm
1991: Honorary doctorate in medicine from Lund University, Sweden
1990: American Association for Cancer Research – G.H.A. Clowes Memorial Award
Member
National Academy of Sciences
National Academy of Medicine
American Academy of Arts and Sciences
European Molecular Biology Organization
Related Disease
Alzheimer’s Disease, Atherosclerosis, Brain Cancer, Breast Cancer, Cancer, Prostate Cancer
The Ruoslahti laboratory studies peptides that home to specific targets in the body, such as tumors, atherosclerotic plaques and injured tissues. These peptides, which usually bind to receptors in the vessels of the target tissue, can be used to selectively deliver diagnostic probes and drugs to the target. The latest development is the discovery of homing peptides with tumor-penetrating properties. The CendR tissue penetration pathway is a new endocytosis/trans-tissue transport pathway (Pang et al., Nat Comm. 2014). The current focus is on enhancing the effects of coupled and co-injected drugs with the tumor-homing peptides, particularly in mouse models of breast cancer and glioblastoma. This laboratory also studies the receptors for the peptides and the mechanism of their tumor penetration activity.
Erkki Ruoslahti’s Research Report
Dr. Ruoslahti’s main scientific contributions are in the field of cell adhesion. He was one of the discoverers of fibronectin. His laboratory subsequently discovered the RGD cell attachment sequence in fibronectin and isolated RGD-directed cellular receptors, now known as integrins. The RGD discovery has led to the development of drugs for diseases ranging from vascular thrombosis to cancer.
Dr. Ruoslahti current studies deal with peptides that specifically target a diseased tissue, particularly its blood vessels. The peptides can be used to deliver drugs and nanoparticles to sites of disease, such as a tumor. The molecules targeted by such disease-specific peptides are of interest regarding their possible role in the disease and potential targets for drug development.
Vascular Zip Codes
The Ruoslahti laboratory screens large collections (“libraries”) of random peptides to identify those that bind to specific targets in tissues. The peptides in the library are displayed on the surface of phage (a virus that infects bacteria), and the screening is done in vivo. When the library is injected into the circulation of a mouse, phage particles that display peptides capable of binding to a selected target tissue, such as a tumor, accumulate at the target where they can be collected and their peptide identified. The process primarily probes the vasculature of the target tissue, unless the vasculature is very leaky. The method has revealed a wealth of specific features, or “vascular zip codes”, in the vessels of individual tissues and tumors. Peptides that specifically home to tumors because they recognize angiogenesis-associated or tumor-type specific markers in tumor blood vessels and can even distinguish the vessels of pre-malignant lesions from those of fully malignant tumors. Homing peptides have also revealed a zip code system of molecular changes in tumor lymphatics.
Synthetic homing peptides have been used to target drugs, biologicals, and nanoparticles into tumors. The targeting can increase the efficacy of a drug while reducing its side effects. Even a non-specifically toxic compound can be converted into a compound that selectively affects the targeted tissue. The peptides make it possible to identify the target molecules (receptors) for the peptides. The receptors of tumor-homing peptides often play a functionally important role in tumor vasculature, and because of this are candidates for drug development.
Tumor-penetrating Peptides
A few years ago the laboratory discovered peptides that not only home to tumor vessels, but are transported through the vascular wall and deep into tumor tissue. The key feature of these peptides is a R/KXXR/K sequence motif, named C-end Rule (CendR) motif or element. In tumor-penetrating peptides, the CendR element is cryptic. These peptides penetrate into tumor tissue in a 3-step process: (i) The peptide binds to a primary receptor on tumor endothelium. In iRGD, the RGD motif recognizes the avb3/avb5 integrins; the primary receptor for the LyP-1 family of peptides is cell surface p32/gC1qR. (ii) The peptide is then cleaved by a protease to expose the CendR element at the C-terminus of the peptide; and, (iii) the CendR element mediates binding to neuropilin-1 (NRP-1), to induce vascular and tissue penetration. The CendR transport pathway triggered through NRP-1 resembles macropinocytosis, but differs from it in being receptor-mediated. Importantly, the responsiveness of the pathway to triggering through NRP-1 is regulated by the nutrient status of cells and tissues. Its physiological function is likely to be to transport nutrients into tissues that lack them. Our ability to trigger the pathway specifically in tumors makes it useful in delivering drugs into tumors.
Targeting the Brain
The Ruoslahti laboratory has recently also applied phage screening to the identification of peptides that target brain diseases. So far, a peptide that specifically recognizes sites of brain injury, and a panel of peptides that are specific for Alzheimer’s brain have been obtained. This topic will be an expanding focus of the laboratory in the near future.
Nanomedicine
A major focus is to use homing peptides as targeting elements to deliver nanoparticles into tumors and other sites of disease. Nanoparticles are considered a promising new approach in medicine because they can be designed to perform more functions than a simple drug. The vasculature is an excellent target for nanoparticles because tumor vessels are readily available for circulating particles. In collaboration with chemistry and bioengineering laboratories, multifunctional nanoparticles for tumor targeting have been constructed. These particles can be directed into tumors in a highly selective manner as demonstrated by histology, non-invasive imaging, and tumor treatment results. The laboratory has constructed nanoparticles with the ability to amplify their own homing to tumors, and are currently working on nanoparticles coated with tumor-penetrating peptides. More recent work has dealt with nanoparticles that target brain diseases or atherosclerotic plaques. The general goal is to engineer nanoparticles with multiple functions. In addition to the specific targeting, such functions include avoidance of the reticuloendothelial system, self-amplification of the targeting, exit from vessels into tissue, ability to send signals for imaging, and controlled drug delivery.
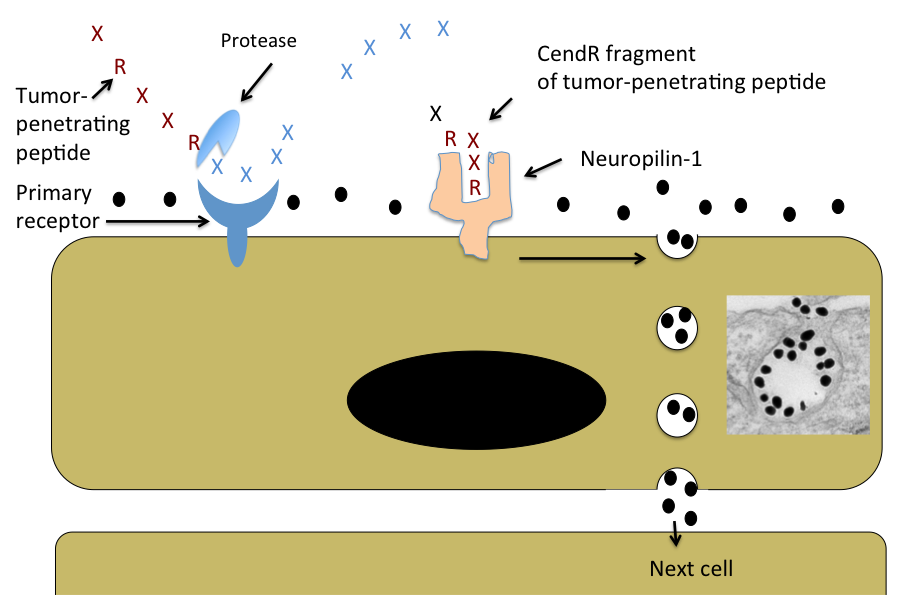
Schematic Representation of the CendR Trans-tissue Transport Pathway
Note that CendR effect enhances the tissue penetration of molecules (depicted here as a black dots) that are co-administered with the peptide, as well as of cargo coupled to the peptide. The inset shows an electron microscopic image of a CendR endocytic vesicle that is budding from the cell surface into the cytoplasm and contains CendR peptide-coated gold nanoparticles (dark dots) See Ruoslahti, Adv. Drug Deliv. Rev. 2016.
Reviews
Ruoslahti E. Tumor penetrating peptides for improved drug delivery. Adv Drug Deliv Rev. 2016 Apr 1. pii: S0169-409X(16)30094-1. doi: 10.1016/j.addr.2016.03.008. Review. PMID: 27040947
Ruoslahti, E. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv. Mat. (review article) 24:3747-3756. (2012). PMCID: PMC3947925
Minoru Fukuda earned his PhD in biochemistry from the University of Tokyo in 1973 and did his postdoctoral training at the Yale University School of Medicine. Following a period with joint appointments at University of Washington and Fred Hutchinson Cancer Research Center in Seattle, he was recruited to Sanford-Burnham Medical Research Institute in 1982 as Director of the Glycobiology Program. Dr. Fukuda directs the program project grant, which consolidates the research efforts of the members of the Glycobiology Program.
Dr. Fukuda is a recipient of a Merit Award from the National Cancer Institute and the 1997 recipient of the Karl Meyer Award from the Society of Glycobiology. He served as an Executive Editor for Biochimica et Biophysica Acta, as an Associate Editor for Cancer Research and Editorial Member for Journal of Biological Chemistry. He also has edited 11 books including three books from Oxford University Press and three volumes of Methods in Enzymology and holds an Adjunct Professor appointment at the University of California, San Diego.
Education
1973: PhD, University of Tokyo, Biochemistry
1970: MS, University of Tokyo, Biochemistry
1968: BS, University of Tokyo, Biochemistry
Related Disease
Brain Cancer, Colorectal Cancer, Gastric Cancer, Helicobacter pylori, Prostate Cancer
The cell surface is heavily coated with carbohydrates. The structure of those cell surface carbohydrates displays a dramatic change during development, and mature cells express cell surface carbohydrates specific to different organs and tissues. Cell surface carbohydrates thus serve as a zip code for different organs and tissues. Sialyl Lewis X represents such an oligosaccharide. After discovery of sialyl Lewis X in neutrophils by Dr. Minoru Fukuda, his laboratory demonstrated that sulfated form of sialyl Lewis X is essential for lymphocyte homing and recruitment of natural killer cells in preventing tumor metastasis to the peripheral lymph node. With colleagues from Japan, Dr. Fukuda discovered that certain carbohydrates function as antibiotics against Helicobacter pylori infection, which is a leading cause for peptic ulcer and gastric carcinoma. Most recent studies in Dr. Fukuda’s laboratory revealed that decrease of the laminin-binding glycans on α-dystroglycan in carcinoma cells leads to tumor cell migration, invasion, and metastasis. The restoration of the unique glycans by the expression of distinct β3-N-acetylglucosaminyltransferase renders these cells act like normal cells. The results indicate that certain carbohydrates on normal cells and enzymes that synthesize those glycans, such as β3-N-acetylglucosaminyltransferase, function as tumor suppressors These findings will be useful in developing carbohydrate-based therapy for the treatment of inflammation and tumor metastasis.
Minoru Fukuda’s Research Report
Cell Surface Carbohydrates as Tumor Suppressor
Many studies have focused on carbohydrates that increase in cancer cells, but only a few have looked at carbohydrates that appear in normal cells but decrease or disappear in cancer cells. A specific mucin-type O-glycans (core 3 O-glycans) is one of such glycans, and we found core 3 O-glycans suppress tumor formation and metastasis. When core 3 O-glycans were forced to express on human prostate cancer cell lines, those prostate cancer cells produced much smaller tumors and almost no metastasis. By contrast, the parent cancer cells, which did not express core 3 O-glycans, produced robust primary and metastatic tumors. We showed that the expression of core 3 O-glycans decreases a formation of α2β1-integrin complex, receptors that mediate cell adhesion, diminishing cancer cell migration.
We also revealed tumor suppressor function in the unique laminin-binding glycans on dystrophin complex, — carbohydrates located on α-dystroglycan, which is also associated with cell adhesion. We discovered that the unique glycans play a critical role in epithelial-basement membrane interaction in normal cells, and the decrease or loss of the glycans, due to downregulation of β3-N-acetylglucosaminyltransferase, leads to increased malignancy by invasive carcinoma cells. Restoration of the laminin-binding glycans by forced expression of β3-N-acetylglucosaminyltransferase, on the other hand, results in reduced cell migration, thus dramatic decrease in tumor formation and metastasis. We demonstrated that interaction of laminin with the unique glycans on α-dystroglycan counteracts the cell migration signals that are mediated by integrin binding to its ligands, thereby decreasing tumor formation and metastasis. These findings also suggest that the laminin-binding glycans can be an excellent marker for epithelial-mesenchymal transitions.
These results indicate that certain carbohydrates on normal cells and enzymes that synthesize those glycans, such as β3-N-acetylglucosaminyltransferase, function as tumor suppressors. Upregulation of those key enzymes may become a novel way to treat cancer.
Neural Cell-specific Glycans in Development and Cancer
Polysialic acid and HNK-1 glycan represent carbohydrates enriched in neural cells. Polysialic acid is mainly attached to NCAM in embryos, while the majority of NCAM in adults lack this carbohydrate. To understand the roles of these glycans in neural development, we have cloned cDNAs encoding human polysialyltransferase, PST, and HNK-1 sulfotransferase, HNK-1ST, that are responsible for the synthesis of polysialic acid and HNK-1 glycan, respectively. By using these cloned cDNAs, we demonstrated that polysialic acid facilitates the invasion of glioma, the most common form of adult brain tumor. Our studies also showed that mutant mice with deficient STX, another polysialyltransferase, exhibit reduced behavioral response to fear conditioning, apparently due to anomalies in mossy fibers of the hippocampus. Our studies also demonstrated that neural development is significantly impaired in mutant mice that entirely lack polysialic acid due to inactivation of two polysialyltransferases. We found that this defect is caused by impairment of neural cell migration.
Mucin-type O-glycans in Immune Cell Interactions
Previously, we found that an increase of core 2-branched oligosaccharides is associated with leukemia and immunodeficiency, such as in Wiskott-Aldrich syndrome and AIDS. To determine the roles of core 2-branched O-glycans in immune cell interactions, the enzyme (C2GnT-1) responsible for the core 2-branched oligosaccharide was knocked-out by gene targeting. Compared to wild-type mice, leukocytes from the gene knockout mice exhibited a reduced binding to L-, E- and P-selectin in this order. In contrast, homing of lymphocytes was moderately reduced. Lymphocyte homing is mediated by binding of L-selectin on lymphocytes to sulfated L-selectin oligosaccharide ligands, 6-sulfo sialyl Lewis X in high endothelial venules (HEV) of secondary lymphoid organs. By analyzing remaining L-selectin ligands in C2GnT-1 knockout mice, we discovered novel L-selectin ligands that are based on extended core 1 oligosaccharides. The core portion of this novel L-selectin ligand is also an epitope for MECA-79 antibody that inhibits lymphocyte homing in vivo. Moreover, crossbreeding between mutant mice with deficient L-selectin ligand sulfotransferase and another sulfotransferase led to our findings that these two enzymes in cooperation synthesize L-selectin ligands. These mutant mice lack 6-sulfate group in L-selectin ligands that results in impaired inflammatory response. More recently, mice deficient in L-selectin ligands on mucin-type O-glycans were generated. The studies on the mutant mice revealed novel functions of N-glycan-based L-selectin ligand, which supports both lymphocyte homing and inflammatory response. This finding brought a new paradigm in selectin-carbohydrate interaction.
Carbohydrate-dependent Adhesion in Tumor Metastasis
Previously we found that the amount of core 2 O-glycans is significantly increased in colon and lung carcinomas and the increase of core 2 O-glycans is highly correlated to vessel invasion and lymph node metastasis. More recently, we discovered that forced expression of core 2 O-glycans by transfecting C2GnT-1 in prostate cancer cell lines resulted in increased tumor formation.
In parallel, we discovered that forced expression of selectin ligands, sialyl Lewis X on B16 melanoma cells leads to increased lung tumor formation. We also showed that tumor formation in lymph node is suppressed by natural killer (NK) cells, which are recruited by L-selectin mediated homing of NK cells to lymph nodes.
Roles of Carbohydrates in Helicobacter pylori-mediated Inflammation and Cancer
Helicobacter pylori is a leading cause of peptic ulcer and gastric cancer. Previously it was shown by others that H. pylori adhere to gastric mucosa in a carbohydrate-dependent manner. The infection of H. pylori leads to chronic inflammation, which apparently leads to peptic ulcer and gastric cancer.
Our recent studies showed that H. pylori-induced inflammation is associated with the formation of peripheral lymph node addressin (PNAd) characterized by binding to MECA-79 antibody and L-selectin. The number of HEV-like vessels expressing PNAd increases as H. pylori-induced inflammation progresses. Moreover, PNAd disappears once H. pyloriis eradicated by antibiotic treatment. These findings indicate that H. pylori-induced inflammation is facilitated by de novo formation of PNAd thereby recruiting lymphocytes. It may be possible to attenuate or prevent the formation of peptic ulcers or gastric cancer by inhibiting L-selectin ligand synthesis, for example by inhibiting the sulfotransferases.
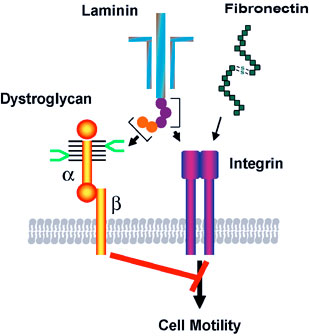
Binding of laminin to the specific carbohydrate (shown in bright green) on α-dystroglycan counteracts the migration signals initiated by integrin binding to extracellular matrix proteins such as laminin and fibronectin. The synthesis of this specific carbohydrate requires a unique β3-N-cetylglucosaminyltransferase, and the downregulation of the glycosyltransferase in carcinoma cells leads to increased cell migration, thereby increased tumor formation and metastasis. Thus the specific carbohydrate structure at cell surface functions as a tumor suppressor, which is controlled by the unique β3-N-acetylglucosaminyltransferase.
While over half of the world’s population is infected with H. pylori, only a fraction of those individuals progress to peptic ulcer and gastric cancer. In relation to these observations, α1,4-N-acteylglucosaminyl capping structure (α4GlcNAc) is present in deeper portions of the gastric mucosa, where H. pylori rarely colonizes. We discovered that α4GlcNAc capping structure functions as an antibiotic against H. pylori infection by inhibition of the synthesis of α-glucosyl cholesterol, a major component of the H. pyloricell wall. This unprecedented discovery should be useful in developing drugs to inhibit H. pylori colonization, through inhibition of cholesterol α-glucosyltransferase. Such drugs lead to a novel treatment for prevention and potential treatment of peptic ulcer and gastric carcinoma.
Education
University College Dublin
BSc and PhD, Pharmacology, cell and molecular biology, signal transduction and cancer, First Class Honors
Related Disease
Brain Cancer, Breast Cancer, Leukemia/Lymphoma, Lung Cancer, Pancreatic Cancer, Skin Cancer and Melanoma
Dr. Finlay’s research is focused on apoptosis (programmed cell death) and how this process can be manipulated to kill cancer cells.
Dr. Wechsler-Reya’s research focuses on the signals that control growth and differentiation in the cerebellum, and how these signals are dysregulated in the brain tumor medulloblastoma. As a postdoc, he demonstrated that Sonic hedgehog (Shh) is a critical mitogen for neuronal precursors in the cerebellum, and that mutations in the Shh pathway predispose to medulloblastoma by activating a mitogenic pathway that normally functions only in early development.
Now in his own lab, he continues to study the relationship between brain development and brain tumor formation. His lab’s contributions include identifying N-myc as a key target of the Shh pathway in neuronal precursors and in tumor cells; discovering a novel population of neural stem cells in the neonatal cerebellum; demonstrating that both neuronal precursors and stem cells can serve as cells of origin for MB; and identifying a population of cancer stem cells that is critical for propagation of Shh-associated tumors.
More recently, Dr. Wechsler-Reya and his group have begun developing new models of medulloblastoma and are using them to test novel therapeutic approaches. His work has garnered several awards, including a Kimmel Scholar Award, an Award for Excellence in Pediatrics Research from the Society for Neuro-Oncology and a Leadership Award from the California Institute for Regenerative Medicine (CIRM).
Education
2001-2010: Associate Professor of Pharmacology and Cancer Biology, Duke University Medical Center
1997-2001: Postdoctoral Fellow, Stanford University, Neural Development
1995-1996: Postdoctoral Fellow, Wistar Institute, Molecular Oncology
1995: PhD, University of Pennsylvania, Immunology
1986: B.A., Harvard College, Psychology & Biology
Funding Awards and Collaborative Grants
Leadership Award from the California Institute for Regenerative Medicine (CIRM)
Other Affiliations
2012: 19th International Brain Tumor Research and Therapy Conference, University of Toronto, Niagara Falls, ON
2012: “Developmental tumors of the nervous system,” held in Barcelona on July 2012, as part of the 8th Forum of European Neuroscience Societies.
Honors and Recognition
2007: W.K. Joklik Award for Excellence in Basic Cancer Research
2007: DukeMed Scholar
2006: Award for Excellence in Pediatrics Research, Society for Neuro-Oncology
2003: Kimmel Scholar Award, Sidney Kimmel Foundation for Cancer Research
2003: Brain Tumor Society Research Award
2002: Children’s Brain Tumor Foundation Research Award
2000-2001: Postdoctoral Fellowship, American Cancer Society (California)
1995-1997: Postdoctoral Fellowship, Medical Research Council of Canada
1988: Award for Excellence in Scientific Writing, American Diabetes Association
1984-1985: John Harvard Scholarship for Academic Achievement of Highest Distinction
Related Disease
Brain Cancer, Childhood Diseases
The Wechsler-Reya Lab studies the signals that control cell growth and differentiation in the nervous system and how these signals are dysregulated in brain tumors. We focus on medulloblastoma, the most common malignant brain tumor in children, and use models to understand the disease and to develop novel approaches to therapy. Our current areas of interest include:
- Discovering oncogenic drivers and creating new models
- Elucidating the molecular mechanisms of metastasis
- Identifying new therapeutics and approaches to drug delivery
- Harnessing the immune system to target tumors
We also work closely with physicians at Rady Children’s Hospital and elsewhere to translate our findings into trials that can benefit patients. Our goal is to develop safer and more effective therapies for children with brain tumors.
Robert Wechsler-Reya’s Research Report
Discovering Oncogenic Drivers and Creating Models
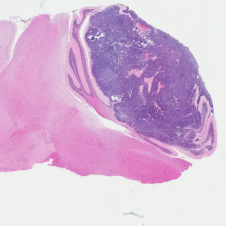
H&E stained Myc-Gfi1 tumor
Aggressive therapies – including surgery, radiation and high-dose chemotherapy – have improved outcomes for medulloblastoma patients, but many patients still die of their disease, and survivors suffer severe long-term side effects from therapy. To develop safer and more effective treatments, we need to understand the genes and pathways that are important for tumorigenesis. But for many forms of medulloblastoma, the oncogenic drivers are still unknown. A major focus of our research is to identify these drivers and use them to create robust animal models of the disease. Sequencing studies have identified genes that are altered in human medulloblastoma, and we are using functional assays to determine which of these genes can promote tumor growth in vivo. We have also established a large bank of patient-derived xenograft models that we use to perturb candidate genes and test their roles in tumorigenesis. In addition to identifying new drivers of medulloblastoma, these studies generate models that can be used to test novel approaches to therapy.
Elucidating the Molecular Mechanisms of Metastasis
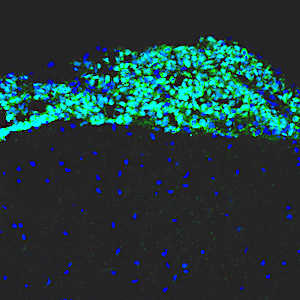
Leptomenigeal metastasis section
Most medulloblastoma research has focused on the primary tumors growing in the cerebellum. However, most medulloblastoma patients do not die from the primary disease, but from leptomeningeal metastasis: the dissemination of tumor cells from the cerebellum into the brain and spinal cord. Metastatic lesions cannot be surgically removed, and there are no effective therapies to eliminate them or stop them from growing. To understand the molecular basis of metastasis, we are using animal models to study the differences between primary and metastatic tumors, and screening for genes that can promote or inhibit metastatic dissemination. We are also integrating our results with molecular data from medulloblastoma patients, to identify genes that are critical for metastasis. Understanding the function of these genes will allow us to design novel strategies for early detection, prevention, and treatment of metastasis in patients with medulloblastoma and other types of brain tumors.
Identifying new Therapeutics and Approaches to Drug Delivery

Tumor-homing peptides in MP tumor
Genomic analyses have revealed that medulloblastoma is an extremely heterogeneous disease, with at least 4 distinct subtypes that differ in terms of mutations, gene expression, epigenetic changes, and patient survival. Despite this heterogeneity, most medulloblastoma patients currently receive the same therapy. A major goal of our research is to discover new therapeutic strategies that are tailored to specific medulloblastoma subgroups. To this end, we have assembled a large panel of patient-derived xenografts and are using them for high-throughput drug screening. Working with experts in genomics and computational biology, we are using statistical and mathematical tools to understand the relationship between molecular alterations and drug responses. These studies not only highlight new targeted therapies for medulloblastoma, but also provide insight into drug response biomarkers and help prioritize agents for clinical trials.
In addition to identifying therapeutics, we are also exploring novel approaches to drug delivery. A major obstacle to treating brain tumors is that the majority of small molecule drugs are not able to cross the blood brain barrier, and those that do are often pumped out by multi-drug transporters. To solve this problem, we are collaborating with bioengineers with expertise in nanotechnology. By encapsulating drugs in nanoparticles and delivering them directly to the central nervous system, we hope to increase the concentration of drugs in brain tumors and reduce the concentrations in other tissues, thereby mitigating systemic side effects. We have already identified several drugs that are effective at killing medulloblastoma cells in vitro; if we can develop strategies for effective delivery of these drugs to tumors, we can markedly improve outcomes for medulloblastoma patients.
Harnessing the Immune System to Target Tumors
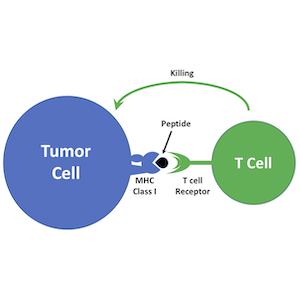
Immunotherapy is emerging as a powerful approach to treating cancer. Antagonists of immune checkpoint regulators, T lymphocytes engineered to recognize tumor antigens, and vaccines that amplify tumor-specific lymphocytes are being tested against a variety of human malignancies. Although some remarkable successes have been reported, only a subset of patients respond to these therapies, and the mechanisms that underlie resistance are poorly understood. Pediatric brain tumors, in particular, have not yet benefited from immunological targeting. We are studying the mechanisms brain tumors use to evade the immune system and suppress immune responses, and developing therapeutic strategies to overcome these mechanisms. We are also using genomic and proteomic approaches to identify antigens that might represent novel targets for vaccines, CAR T cells and natural killer cells. Finally, we are “humanizing” our PDX models so we can explore interactions between the immune system and patient-derived tumor cells. By increasing the immunogenicity of tumor cells and enhancing anti-tumor immune responses, we hope to bring the benefits of immunotherapy to medulloblastoma patients.
 Jul 6, 2022
Jul 6, 2022Heating up cold brain tumors: An emerging approach to medulloblastoma
Jul 6, 2022Immunotherapy has revolutionized cancer treatment, but it doesn’t work on many childhood brain tumors. Researchers from Sanford Burnham Prebys are…
 Apr 7, 2021
Apr 7, 2021Conrad Prebys Foundation provides $3 million for pediatric brain cancer research
Apr 7, 2021Conrad Prebys was an extraordinary man and a passionate philanthropist. Today, his generosity extends beyond his life through the Conrad…
 Dec 14, 2020
Dec 14, 2020Our top 10 discoveries of 2020
Dec 14, 2020This year required dedication, patience and perseverance as we all adjusted to a new normal—and we’re proud that our scientists
 Nov 9, 2020
Nov 9, 2020Personalized drug screens could guide treatment for children with brain cancer
Nov 9, 2020A clinical trial that evaluates the approach for medulloblastoma, the most common malignant pediatric brain tumor, is planned Scientists at…
 Jul 9, 2020
Jul 9, 2020Sanford Burnham Prebys researchers awarded 2020 Padres Pedal the Cause grants
Jul 9, 2020We are pleased to announce that Padres Pedal the Cause (PPTC) has awarded three collaborative research grants to Sanford Burnham…
 Jan 13, 2020
Jan 13, 2020How to help children survive—and thrive—after a brain cancer diagnosis
Jan 13, 2020Lynne Selinka knew in her heart that something was seriously wrong with her 10-year-old son, Travis. For months he had…
Kristiina Vuori earned her MD and PhD at University of Oulu, Finland. After completion of internship and residency, she received postdoctoral training at the Institute and was appointed to faculty in 1996. Dr. Vuori was selected as a PEW Scholar in the Biomedical Sciences in 1997. She has been co-Director of the Conrad Prebys Center for Chemical Genomics, housed at Sanford Burnham Prebys, since its inception in 2005. She was appointed Deputy Director of the Institute’s NCI-Designated Cancer Center in 2003, and Director of the Cancer Center in 2006. In 2008, she was appointed Executive Vice President for Scientific Affairs at Sanford Burnham Prebys. She was President of the Institute from 2010 to 2022.
Related Disease
Brain Cancer, Breast Cancer, Cancer, Leukemia/Lymphoma, Lung Cancer, Ovarian Cancer, Prostate Cancer
Dr. Vuori’s research is aimed at unraveling the cell mechanisms of the most life-threatening aspect of cancer, which is cancer metastasis. Metastasis is responsible for nearly all deaths in cancer patients, and understanding of the mechanisms that turn a cancer from a locally growing tumor into highly metastatic cancer cells will provide clues how to prevent this step in cancer progression. All cells in our body stick to one another and to the packaging material, or extracellular matrix, around them. This adhesion is essential for cell survival; if cells become detached from their microenvironment, they will die through a process known as apoptosis. This phenomenon, which is called adhesion dependency of survival, is one of the safeguards that maintain the integrity and normal function of tissues, and prevent cells from becoming cancerous. Normal cells cannot detach from their tissue and establish themselves somewhere else, because they will die on the way. Yet cancer cells somehow get around this requirement; they trespass aggressively into other tissues and metastasize to distant sites in the body without dying. Dr. Vuori’s work is aimed at identifying the molecular mechanisms that in normal cells makes them adhesion-dependent; false action of the very same mechanisms is likely to be the key step in allowing cancer cells to metastasize.
 Feb 1, 2024
Feb 1, 2024New genome mapping tool may uncover secrets for treating blood cancers
Feb 1, 2024The outlook for patients with acute myeloid leukemia (AML), a deadly set of blood cancers that is difficult to treat,…
 Nov 14, 2017
Nov 14, 2017Cancer Center Open House Showcases SBP Scientists
Nov 14, 2017SBP’s Cancer Center Open House on November 9, 2017 enlightened visitors from the community on the topic of “The Science…
 Oct 18, 2017
Oct 18, 2017Spectacular 2017 SBP annual Gala celebrates “Sights Set on Discovery”
Oct 18, 2017Friends and supporters of Sanford Burnham Prebys Medical Discovery Institute (SBP) gathered under the stars on Harbor Island in downtown…
 Dec 13, 2016
Dec 13, 2016Reena Horowitz honored at Sanford Burnham Prebys Medical Discovery Institute
Dec 13, 2016During a special end-of-the-year gathering, Reena Horowitz was honored for her hard work and dedication to Sanford Burnham Prebys Medical…
 Jun 8, 2016
Jun 8, 2016SBP selected as designated center for Chemical Biology Consortium
Jun 8, 2016SBP has been chosen as a dedicated center for the Experimental Therapeutics (NExT) Program Chemical Biology Consortium (CBC), centered at…
 Sep 1, 2015
Sep 1, 2015The Mayor of San Diego visits SBP in La Jolla
Sep 1, 2015On Friday, August 28, the Mayor of San Diego, Kevin Faulconer, visited SBP to learn more about how our Institute is…
Xueqin (Sherine) Sun seeks to better understand the genetic and epigenetic underpinnings of cancers, using genome editing technologies, animal and patient-derived models, and other tools to develop more effective cancer therapies.
“My lab is interested in studying how DNA or the machinery that interprets it leads to the transformation of normal cells into cancerous cells and concurrently, their specific vulnerabilities. Identifying these intrinsic vulnerabilities and targeting them properly is profoundly important to developing effective cancer therapies.”
Another aspect of Sun’s work is understanding how cancer cells and tumors change their circumstances and environment to improve survival, including hiding from or repressing the immune system.
“Changes to DNA itself and the way how DNA is interpreted by cells can transform normal cells into cancer cells. And transformed cells propagate by enhancing the misinterpreted DNA information, which in turn becomes the Achilles’ heel of cancer cells. Our goal is to find out how DNA information is misinterpreted in different ways and how to correct it to halt cancer.”
At Sanford Burnham Prebys, Sun and colleagues will employ a host of leading-edge tools and approaches, including functional genomics, artificial intelligence, structural biology, large-scale drug screening, and advanced imaging/spatial technologies.
Sun conducted her postdoctoral fellowship at Cold Spring Harbor Laboratory under the guidance of Alea Mills, PhD, a professor at the National Cancer Institute-designated cancer center at Cold Spring Harbor.
She received her PhD from Wuhan University in China.
Related Disease
Aging-Related Diseases, Brain Cancer, Cancer, Childhood Diseases, Immune Disorders, Inflammatory/Autoimmune Disease, Leukemia/Lymphoma
Phenomena or Processes
Adapter Proteins, Adult/Multipotent Stem Cells, Aging, Angiogenesis, Apoptosis and Cell Death, Bcl-2 Family, Cancer Biology, Cancer Epigenetics, Cell Adhesion and Migration, Cell Biology, Cell Cycle Progression, Cell Differentiation, Cell Motility, Cell Proliferation, Cell Signaling, Cell Surface Receptors, Cellular Senescence, Chromosome Dynamics, Combinatorial Therapies, Cytokines, Development and Differentiation, Disease Therapies, DNA Damage Checkpoint Function, Embryonic/Pluripotent Stem Cells, Epigenetics, Gene Regulation, Genomic Instability, Growth Factors, Hematopoiesis, Host Defense, Host-Pathogen Interactions, Inflammation, Innate Immunity, Kinase Inhibitors, Metastasis, Neurogenesis, Oncogenes, Phosphorylation, Posttranslational Modification, Receptor Tyrosine Kinases, Serine/Threonine Kinases, Signal Transduction, TNF-Family, Transcription Factors, Transcriptional Regulation, Tumor Microenvironment, Tumorigenesis, Tyrosine Kinases, Ubiquitin, Ubiquitin Protease System and Ubiquitin-like Proteins
Anatomical Systems and Sites
Brain, General Cell Biology, Hematopoietic System, Immune System and Inflammation, Nervous System
Research Models
Bacteria, Cultured Cell Lines, Human Adult/Somatic Stem Cells, Human Cell Lines, Mouse, Mouse Cell Lines, Mouse Embryonic Stem Cells, Mouse Somatic Stem Cells, Primary Cells, Primary Human Cells
Techniques and Technologies
3D Image Analysis, 3D Reconstructions, Biochemistry, Bioinformatics, Cell Biology, Cellular and Molecular Imaging, Chemical Biology, Computational Biology, Confocal Microscopy, Correlative Light and Electron Microscopy, Drug Delivery, Drug Discovery, Drug Efficacy, Electron Microscopy, Fluorescence Microscopy, Fragment-Based Drug Design, Gene Expression, Gene Knockout (Complete and Conditional), Gene Silencing, Genetics, Genomics, High Content Imaging, High-Throughput/Robotic Screening, In vivo Modeling, Live Cell Imaging, Live Imaging, Mass Spectrometry, Microscopy and Imaging, Molecular Biology, Molecular Genetics, Nucleic Acid Synthesis, Protein-Protein Interactions, Protein-Small Molecule Interactions, Proteomics, Rational Drug Design, RNA Interference (RNAi), Scanning Cytometry, Small Molecule Compounds, Transgenic Organisms, Transplantation
We seek to understand why cancer occurs and what is the Achille’s heel of cancer, and to develop effective therapeutic interventions.
The successful treatment of any disease requires a good understanding of the mechanisms at work. Cancer is fundamentally caused by aberrant gene expression, which reflects the misinterpretation of DNA information at both genetic and epigenetic levels. We are interested in uncovering DNA-related alterations that drive cancer-favored transcriptional programs, identifying cancer-specific vulnerabilities, and developing effective therapeutic interventions for cancer treatment.
Xueqin Sun’s Research Report
Precise gene expression (the interpretation of DNA) is essential for almost all biological processes, and understanding gene regulation is one of the most pivotal frontiers in biological research under both health and disease circumstances. Gene expression is mainly regulated at genetic (with changes of DNA sequence) and epigenetic (without changing DNA sequence) levels. And gene dysregulation can lead to various health conditions and diseases, including developmental disorders, aging, and cancer. The overarching goal of Sun Lab is to uncover driving genetic and epigenetic alterations involved in cancer, to understand how developmental pathways and aging process impact cancer progression, and to identify mechanisms of action for developing more effective therapeutic strategies.
We are an interdisciplinary lab particularly focused on the following research directions:
- The EP400 chromatin remodeling complex
The EP400 complex is an evolutionarily conserved SWR1-class ATP-dependent chromatin remodeling complex encompassing ~17 components, with a total molecular mass of ~1.5 mega-dalton. The EP400 complex plays critical roles in diverse cellular processes, including chromosome stability, transcription, DNA recombination, DNA damage repair, embryonic stem cell renewal/development, and oncogenesis. The EP400 complex can incorporate histone variants, such as H2AZ and H3.3, into the genome to regulate gene expression. Our recent work discovers BRD8—one of the core subunits of the EP400 complex—as a unique vulnerability of p53 wildtype glioblastoma (GBM), the most prevalent and devastating type of brain cancer. BRD8-driven EP400 complex highjacks H2AZ at p53 target loci to block p53-mediated transactivation and tumor suppression (Nature, 2023). The bromodomain of BRD8 plays the key role in this process. Bromodomain is a druggable domain as evidenced by a number of successful small molecules targeting diverse bromodomains encoded by the human genome across multiple cancer types. Furthermore, findings from others and us suggest that the EP400 complex is involved in different cancers. Thus, we seek to unravel the roles of the EP400 complex in health and disease, and to better understand how to target the EP400 complex for developing effective therapeutic interventions. - The NuRD chromatin remodeling complex
The NuRD complex is also a highly conserved class of ~ 1 MDa multi-subunit chromatin remodeling complexes that consume energy derived from ATP hydrolysis to remodel the configuration of chromatin to control gene transcription programs, with a primary role in gene silencing. Chromatin remodeling is vital for efficiently framing the cellular response to both intrinsic and extrinsic signals and has enormous implications for determining cellular states. NuRD complex is unique in combining ATP-dependent chromatin remodeling, protein deacetylase activity, and recognition of methylated DNA and histone modifications, and has multifarious roles in chromatin organization, transcription regulation, and genome maintenance; thereby, largely impacts health and disease. The NuRD complex has been in the central stage of brain development studies, and is significantly related to brain disorders/diseases. Interestingly, NuRD complex re-assembles by exchanging the chromatin remodeling subunits CHD3/4/5 to achieve specific regulation of an array of genes required for generating distinct cell types in a highly organized manner, especially over brain development. Amongst the genes encoding NuRD complex components, CHD5 is located in human chromosome 1 short arm (1p36), a region that is frequently hemizygously deleted in diverse cancers. Besides genetic deletion, CHD5 is also often silenced in cancer cells due to epigenetic mechanisms, such as promoter hypermethylation, aberrant expression of other chromatin regulators, and microRNAs-mediated translational repression and/or mRNA instability. Our current work seeks to determine whether and how CHD5-driven NuRD complex is involved in tumorigenesis (In preparation, 2024). We will further understand how NuRD complex is involved in both development and tumorigenesis, and identify mechanism of action to develop rational therapeutic strategies. - Novel genetic and epigenetic underpinnings in GBM
GBM is notorious for being a highly complex and plastic cancer type. However, at the genetic level, GBM harbors a relatively low genetic alteration burden compared to the majority of other cancers from pan-cancer profiling studies. This indicates the largely undocumented epigenetic mechanisms that interplay with genetic alterations and co-reprogram transcriptional networks essential for GBM development. Epigenetic changes are usually reversible by nature, as evidenced by numerous successes in targeting epigenetic regulators using small chemical compounds. As actionable therapeutic targets for GBM have been scarce, we are keen to uncover novel epigenetic pathways underlying gliomagenesis under different genetic backgrounds, which will potentially provide promising therapeutic opportunities for GBM treatment. - Novel GBM mouse models
Despite decades of effort, our knowledge about GBM biology is still very limited. GBM harbors a number of genetic alterations. However, among these recurrent genetic lesions, only several have been implicated in gliomagenesis, with most being undocumented. Moreover, the mechanisms by which these genetic alterations are involved in establishing GBM-favored epigenetic landscapes and transcription programs during GBM progression are still largely elusive. The lack of efficient approach to establish mouse models for investigating gene function in gliomagenesis and the limit of current mouse models to recapitulate clinical GBM features in brain is the prime reason that hinders GBM biological research. To this end, we have developed an engineered neural stem cells (NSCs)-based strategy to rapidly generate highly aggressive GBM with desired genetic lesions (genotypes) in mouse brain. Therefore, we will further optimize this strategy to establish a series of novel mouse models possessing recurrent combinations of genetic alterations (genotypes) in GBM, in order to systematically study whether and how these genetic lesions are involved in gliomagenesis and identify genotype-specific dependencies. - Crosstalk between GBM cells and tumor microenvironment
GBM exhibits highly diffuse and infiltrative nature, which contributes to therapeutic resistance and tumor relapse after surgical removal, resulting in dismal prognosis. A better understanding of gliomagenesis involving not only malignant cells themselves, but also the holistic bidirectional interactions of malignant cells with a variety of proximal and distal cells within the organism, is profound for developing novel effective therapies to improve GBM prognosis. Individual invasive GBM cells intermingle with normal brain cells and often cause relapse in brain areas essential for patient survival. Emerging evidence indicates that glioma cells highjack normal brain cells to thrive, and even transform them. However, how gliomagenesis reshapes ecological composition/landscape in host brain and how brain microenvironment affects gliomagenesis are still largely unclear. By using our novel highly invasive mouse models that recapitulate the multiforme diffuse topographies of GBM in brain, we seek to understand the interactions between GBM cells and brain microenvironment, and identify extrinsic pathways that are essential for GBM progression and migration.
Our lab is focused on both fundamental questions in cancer biology and translation of promising therapeutic strategies.
To achieve these, we work together with many fantastic collaborators to develop and leverage cutting-edge technologies, including but not limited to, high-throughput functional genomics (CRISPR/Cas9 screens, exon tiling scan, targeted mutagenesis, etc.), cell and molecular biology, genomics, epigenomics, proteomics, biochemistry, microscopy (2D/3D, time-lapse, two-photon, light sheet, etc.), automated large-scale drug synthesis/screening, structural biology, single cell and spatial multi-omics, artificial intelligence, and bioinformatics. We also establish novel patient-derived models and novel mouse models to facilitate our research programs. Our ultimate goals are to better understand fundamental genetic and epigenetic apparatuses involved in cancer-specific transcriptional networks, provide more effective therapeutic opportunities, and contribute to shifting the paradigms in cancer treatment and precision medicine.
 Dec 12, 2025
Dec 12, 2025Sanford Burnham Prebys scientists garner eight cancer research grants from Curebound to advance therapeutic treatments and cures
Dec 12, 2025Ten scientists at Sanford Burnham Prebys Medical Discovery Institute were awarded eight grants yesterday from Curebound, a San Diego-based philanthropic organization
 Sep 29, 2025
Sep 29, 2025Xueqin Sun awarded $600,000 V Foundation grant to study one of the most common and deadly brain cancers
Sep 29, 2025The new award will fund research regarding a hidden weakness in glioblastoma tumors that could lead to a new treatment
 Aug 19, 2024
Aug 19, 2024Women in Science event at Sanford Burnham Prebys examines how female faculty members navigate research careers
Aug 19, 2024Topics at the event included work/life balance, caregiving and family obligations, and gender disparities in academic rank at research and…
 Mar 13, 2024
Mar 13, 2024Xueqin Sun seeks to illuminate the underlying causes of cancer
Mar 13, 2024New Sanford Burnham Prebys scientist investigates the mutational powers of cancer cells — and their vulnerabilities
 Dec 19, 2023
Dec 19, 2023Sanford Burnham Prebys continues unprecedented recruitment of early-career scientists
Dec 19, 2023Continuing its rapid and dramatic recruitment of emerging, top-tier researchers, Sanford Burnham Prebys has hired two more highly regarded early-career…
Evan Y. Snyder earned his MD and PhD (in neuroscience) from the University of Pennsylvania in 1980 as a member of NIH’s Medical Scientist Training Program (MSTP). He had also studied psychology and linguistics at the University of Oxford. After moving to Boston in 1980, he completed residencies in pediatrics and neurology as well as a clinical fellowship in Neonatal-Perinatal Medicine at Children’s Hospital-Boston, Harvard Medical School. He also served as Chief Resident in Medicine (1984-1985) and Chief Resident in Neurology (1987) at Children’s Hospital-Boston. In 1989, he became an attending physician in the Department of Pediatrics (Division of Newborn Medicine) and Department of Neurology at Children’s Hospital-Boston, Harvard Medical School. From 1985-1991, concurrent with his clinical activities, he conducted postdoctoral research as a fellow in the Department of Genetics, Harvard Medical School. In 1992, Dr. Snyder was appointed an instructor in neurology (neonatology) at Harvard Medical School and was promoted to assistant professor in 1996. He maintained lab spaces in both Children’s Hospital-Boston and at Harvard Institutes of Medicine/Beth-Israel Deaconess Medical Center. In 2003, Dr. Snyder was recruited to Sanford Burnham Prebys as Professor and Director of the Program in Stem Cell and Regenerative Biology. He then inaugurated the Stem Cell Research Center (serving as its founding director) and initiated the Southern California Stem Cell Consortium. Dr. Snyder is a Fellow of the American Academy of Pediatrics (FAAP). He also received training in Philosophy and Linguistics at Oxford University.
Related Disease
Alzheimer’s Disease, Amyotrophic Lateral Sclerosis (Lou Gehrig’s Disease), Arthritis, Brain Cancer, Brain Injury, Breast Cancer, Cancer, Childhood Diseases, Congenital Disorders of Glycosylation, HIV-Associated Dementia, Huntington’s Disease, Multiple Sclerosis, Muscular Dystrophy, Neurodegenerative and Neuromuscular Diseases, Neurological and Psychiatric Disorders, Parkinson’s Disease, Peripheral Vascular Disease, Skin Cancer and Melanoma, Spinal Cord Injury, Stroke, Traumatic Injury
We believe the study of stem cell biology will provide insights into many areas: developmental biology, homeostasis in the normal adult, and recovery from injury. Indeed, past and current research has already produced data in these areas that would have been difficult or impossible via any other vehicle. We have engaged in a multidisciplinary approach, simultaneously exploring the basic biology of stem cells, their role throughout the lifetime of an individual, as well as their therapeutic potential. Taken together, these bodies of knowledge will glean the greatest benefit for scientists and, most importantly, for patients. All of our research to date has been preformed in animal models with the ultimate goal of bringing them to clinical trials as soon as possible. Stem cells offer an intriguing mix of controversy, discovery, and hope. Politicians are charged with dealing with the controversial facets of stem cells, as we prefer to focus our energy on their potential for discovery and hope.
The Snyder Lab studies stem cell biology, with the goal of understanding normal development, tissue homeostasis, and recovery from injury and disease. A major focus is neural stem cells (NSCs), which can self-renew and differentiate into neurons, astrocytes, and oligodendrocytes. These properties make NSCs ideal for repair of damage due to injury or disease, but they also make them susceptible to transformation into malignant cancers.
 May 28, 2025
May 28, 2025Evan Snyder named Fellow of the Child Neurology Society
May 28, 2025Evan Snyder, MD, PhD, has been named a Fellow of the Child Neurology Society, honoring a distinguished career and…
 Nov 11, 2024
Nov 11, 2024Seven questions for FDA advisor Evan Snyder
Nov 11, 2024Sanford Burnham Prebys physician-scientist advises the FDA on cell-based therapeutics, tissue engineering and gene therapies.
 Jul 23, 2024
Jul 23, 2024Mini lungs make major COVID-19 discoveries possible
Jul 23, 2024Scientists infect miniature lung organoids with the virus responsible for COVID-19, revealing new ways in which the infection spreads and…
 Jan 25, 2022
Jan 25, 2022New CIRM grant to fund research internships for underrepresented high school students
Jan 25, 2022Thanks to a new grant awarded to Sanford Burnham Prebys by the California Institute for Regenerative Medicine (CIRM), 57 California…
 Nov 18, 2021
Nov 18, 2021From child neurology to stem cells: An interview with Evan Snyder
Nov 18, 2021What do Evan Snyder and Sigmund Freud have in common? Both radically changed how we see the human brain. In…
 Jul 26, 2021
Jul 26, 2021Biomarker could help diagnosis schizophrenia at an early age
Jul 26, 2021Research could lead to blood-based diagnostic test Scientists at Sanford Burnham Prebys have discovered how levels of a protein could…
Lukas Chavez is an Associate Professor at the Sanford Burnham Prebys. He is also the Director of the Clayes Research Center for Neuro-Oncology at the Institute for Genomic Medicine at the Rady Children’s Hospital, San Diego. In this role, he works with a team of physicians and scientists to capture genomic, transcriptomic, epigenetic and functional data from pediatric brain tumor patients, and uses this information to improve diagnosis and treatment. His research interests focus on structural variants as well as circular extrachromosomal DNA (ecDNA) in childhood cancers. These extrachromosomal DNA circles are frequently found in highly aggressive solid tumors and represent a new target for improved therapeutic approaches.
Education
2010: PhD, Free University, Berlin
Honors and Recognition
2020: St. Baldrick’s Scholar Award, St. Baldrick’s Foundation
2019: Award of Excellence in Pediatric Neuro-Oncology, Society of Neuro-Oncology
2012–2015: Feodor-Lynen Fellowship for Postdoctoral Researchers, Alexander-von-Humboldt Foundation
Related Disease
Brain Cancer, Cancer
Phenomena or Processes
Cancer Biology, Cancer Epigenetics, Chromosome Dynamics, Combinatorial Therapies, Gene Regulation, Genomic Instability, Oncogenes, Transcriptional Regulation
Anatomical Systems and Sites
Brain
Research Models
Clinical and Transitional Research, Computational Modeling, Cultured Cell Lines, Human, Human Cell Lines, Mouse
Techniques and Technologies
Bioinformatics, Cell Biology, Computational Biology, Computational Modeling, Gene Expression, Gene Silencing, Genomics, Single Nucleotide Polymorphisms (SNPs)
 Dec 12, 2025
Dec 12, 2025Sanford Burnham Prebys scientists garner eight cancer research grants from Curebound to advance therapeutic treatments and cures
Dec 12, 2025Ten scientists at Sanford Burnham Prebys Medical Discovery Institute were awarded eight grants yesterday from Curebound, a San Diego-based philanthropic organization
 Aug 15, 2025
Aug 15, 2025Zombie cancer cells give cold shoulder to chemotherapy
Aug 15, 2025Study shows that combining chemotherapy with targeted treatment for these lumbering cells improves outcomes in mice.
 Aug 13, 2025
Aug 13, 2025Chavez joins NIH Cancer Genetics Study Section
Aug 13, 2025Lukas Chavez, PhD, has been named a standing member of the Cancer Genetics Study Section at the National Institutes…
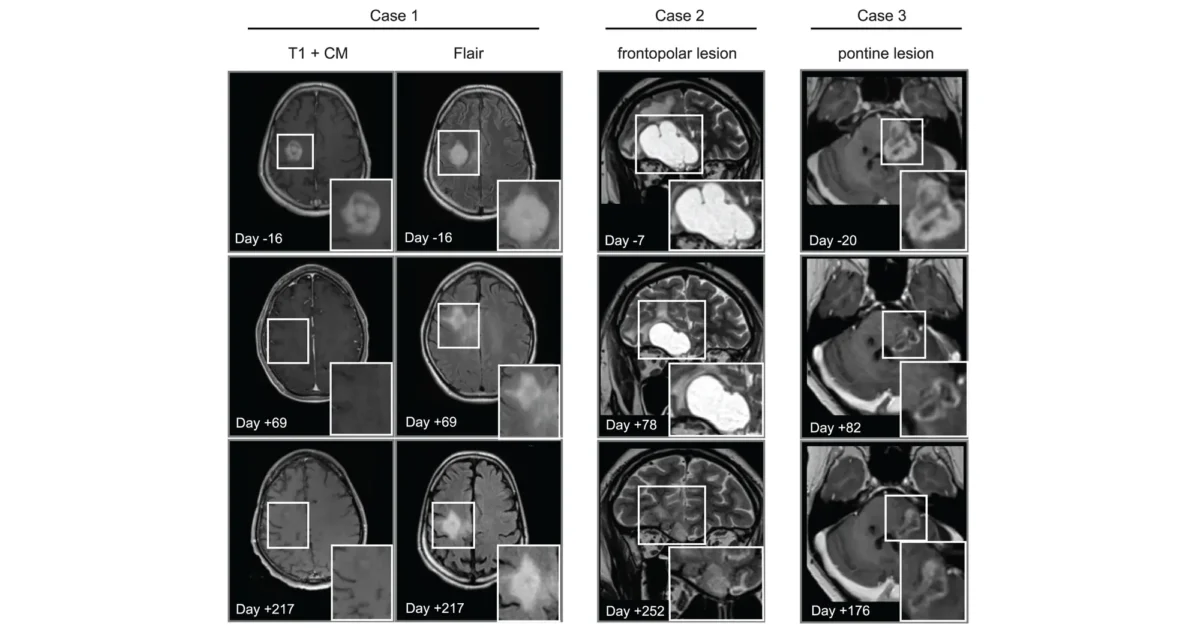 Apr 14, 2025
Apr 14, 2025Cancer drug finds new purpose in the brain
Apr 14, 2025Scientists show that a cancer drug travels to and shrinks some brain tumors, which may lead to new therapies.
 Aug 6, 2024
Aug 6, 2024Coding clinic
Aug 6, 2024Rapidly evolving computational tools may unlock vast archives of untapped clinical information—and help solve complex challenges confronting healthcare providers
 Jul 30, 2024
Jul 30, 2024Using machines to personalize patient care
Jul 30, 2024Artificial intelligence (AI) and other computational techniques are aiding scientists and physicians in their quest to create treatments for individuals…