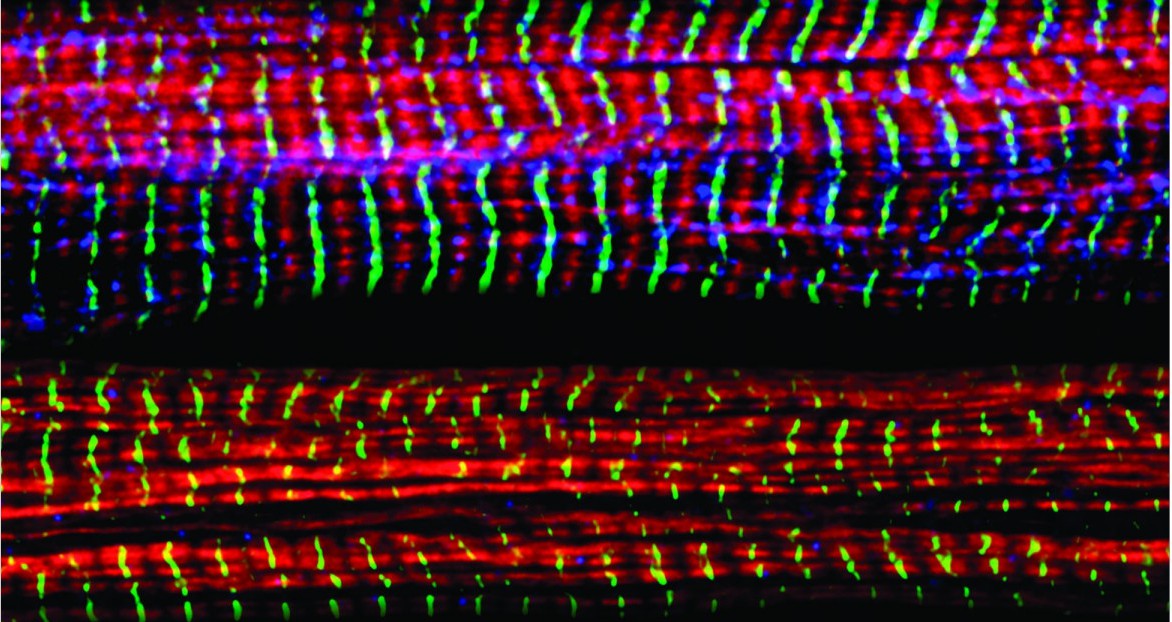
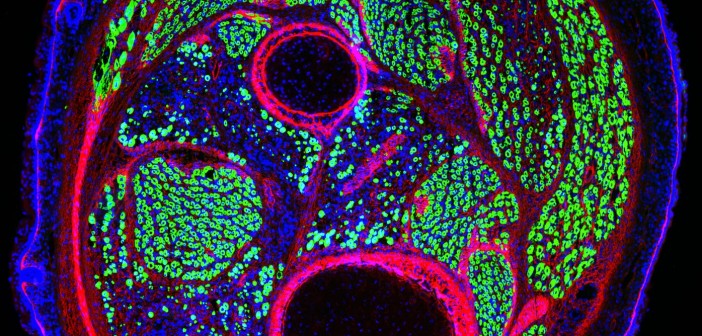
Nearly 30 years of discoveries by a Sanford Burnham Prebys scientist and collaborators lead to federal approval of the first non-steroidal drug to treat Duchenne muscular dystrophy.
For one San Diegan scientist at Sanford Burnham Prebys, the March 2024 federal approval of a new drug to treat Duchenne muscular dystrophy (DMD) marked a milestone in three decades of studying muscle regeneration and muscle-wasting diseases.
The compound, called Givinostat and marketed as DUVYZAT™, is a histone deacetylase inhibitor (HDACi) and was approved by the FDA for the treatment of boys with DMD.
“I have been working from the very beginning of my research career to translate early, basic discoveries into a treatment for DMD,” said Pier Lorenzo Puri, MD, director and professor in the Development, Aging and Regeneration Program at Sanford Burnham Prebys. “The lack of effective treatments for boys with DMD has left families and patients hopeless since the discovery of this disease. Witnessing the progression of such a disease without any option to counter its progression is cruel and I felt the urgency to help these people.”
DMD is the most frequent form of muscular dystrophy affecting approximately 1 in 3,500 male births. DMD is linked to the chromosome X, which harbors the gene coding the protein called dystrophin. As such, the disease develops only in males receiving from their mother the X chromosome carrying mutations in the dystrophin gene that impair production of dystrophin. Dystrophin is a protein that protects muscles from degeneration after they contract and relax. In its absence, the muscles of boys with DMD are prone to damage and undergo cycles of contraction and degeneration that eventually lead to muscle wasting and reduced function.
For decades, steroids have provided the standard-of-care for DMD, but steroids represent an empirical and palliative treatment based on their general anti-inflammatory properties, rather than a treatment that targets specific pathological events that contribute to DMD progression. Moreover, the chronic use of steroids is complicated by many side effects, including weight gain, weak bones, high blood pressure and behavior changes.
Puri decided to focus on the potential use of HDACi to treat DMD after he made seminal basic discoveries that revealed how muscle growth and regeneration are regulated by two enzymes with opposing activities: histone acetyltransferases and deacetylases.
“HDACi are not going to cure muscular dystrophy, but they do provide the first pharmacological treatment that can delay DMD progression, regardless of the type of mutation, and do so in a financially affordable way,” said Puri.
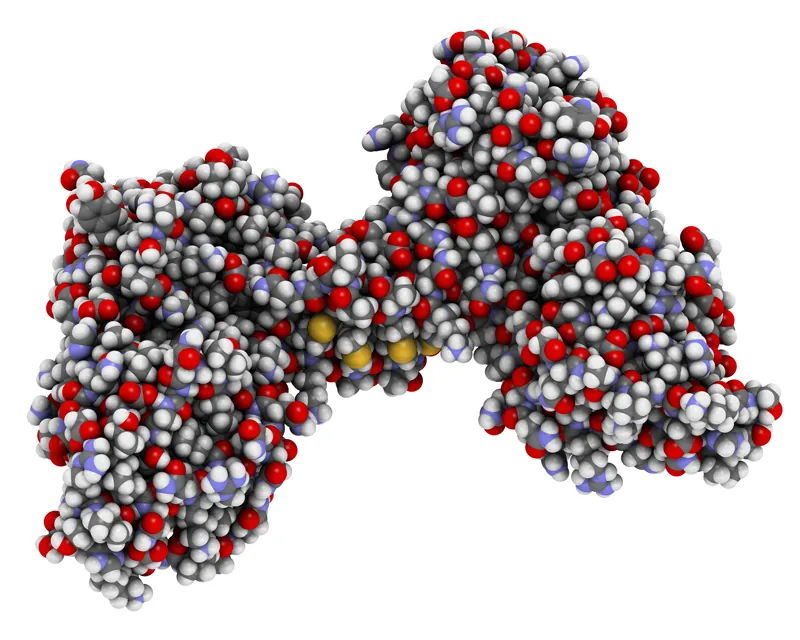
The molecular structure of dystrophin, a protein that protects muscles from degeneration after they contract and relax.
“It is important to note that FDA approval of Givinostat is not the end of the journey, but the beginning. The very good news here is that there is room to improve the efficacy of HDACi-based treatment for DMD by using already existing compounds or by developing novel molecules endowed with an improved therapeutic potential. This is because Givinostat has been used at sub-optimal concentrations due to potential adverse effects, and this might have limited its efficacy as a HDACi.
“People ask me whether Givinostat was approved because it is the most effective molecule among existing HDACi. The answer is that Givinostat has been the first, and so far the only, HDACi to be tested in clinical trials for boys with DMD. Givinostat might not entirely express the therapeutic potential of HDACi for DMD. A reasonable and exciting goal of future studies is to identify HDACi that surpass Givinostat in terms of therapeutic efficacy.”
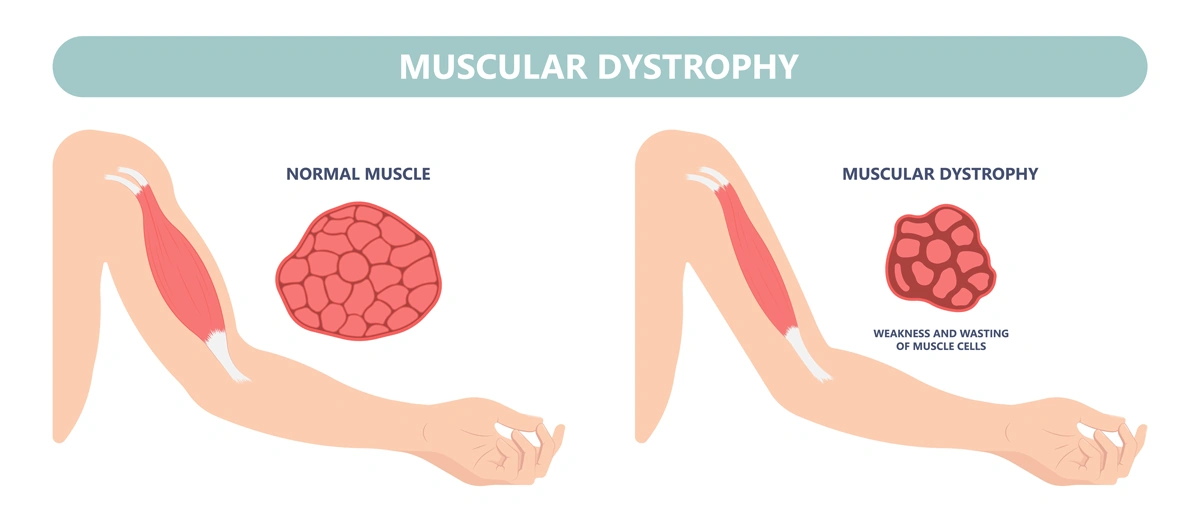
Without dystrophin, the muscles of boys with Duchenne muscular dystrophy are prone to damage and undergo cycles of contraction and degeneration that eventually lead to muscle wasting and reduced function.
Puri noted that, “It is also important to carefully investigate functional interactions between HDACi and steroids, as the clinical trial with Givinostat has been performed while research participants were receiving steroids. However, because the activity of steroids is largely dependent on HDAC, it is very likely that these two treatments could collide, rather than synergize in producing beneficial effects on the muscles of DMD boys.”
He also emphasized that the impact of FDA approval of Givinostat extends way beyond the possibility to offer a treatment for DMD.
“It is the first evidence that it is possible to treat DMD by targeting pathogenic events induced as a consequence of dystrophin deficiency, as an effort parallel to gene and cell therapy, which will hopefully converge into future and more effective combined therapies.”
Building the basic science foundation
Puri’s contributions to the approval of the first non-steroidal drug to treat DMD span nearly 30 years of basic and preclinical research of diseases thought to be incurable — especially pediatric conditions he thought especially cruel.
The puzzle pieces began to take shape as Puri was studying the growth of muscle cells and skeletal muscle tissue, a biological process called skeletal myogenesis. He started working on this project in the 1990s in the laboratory of molecular biology directed by Massimo Levrero, MD, in Rome. Puri had earned his medical degree in 1991 before conducting an internship in Internal Medicine.
“I started there, in a lab inside a hospital in Rome,” said Puri. “I used to see patients until the early afternoon and then I was running to the lab to perform experiments. As a young clinician with a passion for basic research, I was always developing experiments with patients in mind.”
Puri decided to test his first hypothesis by using what was then an innovative technology called microinjection to insert DNA or antibodies inside cultured cells. He decided to spend several months at the Free University of Berlin in the laboratory of Adolf Graessmann, PhD, a pioneer of this technique. Puri later decided to go to the United States to work in the lab of Jean Y.J. Wang, PhD, at the University of California San Diego, to further develop his studies.
After entering the U.S., Puri has worked closely with his long-term collaborator and friend Vittorio Sartorelli, MD, currently the deputy scientific director of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and chief of the institute’s Laboratory of Muscle Stem Cells & Gene Regulation. Together, they uncovered an important role for two groups of enzymes, histone acetyltransferases and deacetylases, that control access to DNA by altering the structure of chromatin.
Histone acetyltransferases control DNA accessibility by adding acetyl groups to histones, which loosens the wrapping of DNA around them, essentially ‘opening’ chromatin and promoting gene expression. Histone deacetylases reverse the process by removing acetyl groups, limiting the activity of genes and the production of key proteins.
“One of our seminal findings was the discovery of associations between myogenesis and an enzymatic activity that could be pharmacologically modulated,” explains Puri.

Puri with current members of his lab at Sanford Burnham Prebys.
Puri and Sartorelli started to explore the possibility that pharmacological modulators of this process by HDACi could affect the growth of muscle cell progenitors and their ability to form contractile muscle tissues.
After finding that HDACs limit muscle cell differentiation, the team’s next step was to find compounds (inhibitors) capable of blocking HDACs from removing acetyl molecules and reducing myogenesis. “We decided see if there is an HDAC inhibitor — an inhibitor of the inhibitor — because this could reduce muscle loss,” notes Puri. “There were a few compounds, and we found a strong effect every time we tested them.”
“I remember vividly the first time I saw the effect of HDACi on cultured muscle cells. I was a postdoc in Jean Wang’s lab at UC San Diego, and one morning I opened the incubator and found that muscle cells treated with HDACi had formed giant myotubes (the contractile muscle). Of course, we started to wonder whether such evidence could provide the rationale we were looking for. If so, this could pave the way toward discovering pharmacological interventions that may promote muscle regeneration.

Puri with Chiara Mozzetta, PhD, faculty at the Institute of Molecular Biology and Pathology at the National Research Council of Italy. Mozzetta previously conducted a postdoctoral fellowship in the Puri lab and made major contributions to the discovery of HDAC inhibitors as therapeutics for DMD.
“But, at that time, it sounded more like a dream. We did not even dare to imagine that the final outcome would have been the identification of a pharmacological treatment for DMD.”
Pursuing the preclinical potential
Puri and Sartorelli pursued their dream, driven by encouraging experimental evidence and discoveries. Still, it took years to provide the rationale for testing HDACi in DMD—years and a few fortunate coincidences. One was the identification of follistatin as a mediator of the action of HDACi on muscle cells. Follistatin is the endogenous inhibitor of myostatin, a potent inhibitor of muscle growth and size, ensuring that muscles do not grow too large.
It was during that time that other groups independently discovered that genetic mutations in the myostatin gene resulted in an abnormal increase in muscle mass in cattle, mice and humans. More importantly, it was published that genetic or pharmacological inactivation of myostatin could exert beneficial effects in a mouse model of DMD.
“That discovery suggested the rationale of testing whether HDACi could exert similar beneficial effect in the same mouse model of DMD,” said Puri. The team hypothesized that this could be achieved by using HDACi to block myostatin activity through the induction of follistatin.
Puri and Sartorelli decided to treat mdx mice — the mouse model of DMD — with a few HDACi. Puri performed these studies with a group of investigators that worked synergistically in his two labs (one in San Diego and the Dulbecco Telethon Institute laboratory in Rome) and in collaboration with Italian scientists Carlo Gaetano, PhD, and Claudia Colussi, PhD.
“The results of the experiment went beyond the most optimistic expectations,” said Puri. “The muscles were bigger. There was no scar tissue, no abnormal fatty deposits and less inflammation. The treated mice were running like normal mice.”
Puri and his collaborators published results in Nature Medicine in 2006 demonstrating significant improvements in muscle composition and exercise performance.
In follow-up studies, Puri and collaborators realized that the therapeutic properties of HDACi extended beyond the targeting of follistatin/myostatin interactions. The pace of discoveries increased along with the improved knowledge on DMD pathogenesis. Key and timely information was gained through identification of a population of muscle interstitial cells, called fibro-adipogenic progenitors (FAPs), from the laboratories of Fabio Rossi, MD, PhD, at the University of British Columbia in Canada and Dr. Kunihiro Tsuchida, MD, PhD, at Fujita Health University in Japan.
FAPs support muscle regeneration of normal muscles, but in dystrophin-deficient muscles these cells turn into the main effectors of fibrotic scars and fat infiltration — the most deleterious events in DMD pathogenesis. Studies from the Puri lab demonstrated that HDACi could restore the ability of FAPs from DMD muscles to promote regeneration, while blocking their pro-fibrotic and adipogenic activity.

Puri with collaborator Vittorio Sartorelli, MD, the deputy scientific director of the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
“The identification of FAPs was a lucky coincidence, as it provided one of the main cell types targeted by HDACi and enabled the identification of the molecular mechanism of action that accounts for the therapeutic properties of HDACi,” said Puri.

Puri (at right) with collaborator Fabio Rossi, MD, PhD, professor of Medical Genetics at the University of British Columbia in Canada, at a muscle research meeting in Montreal in July 2024.
“It also helped to set in our preclinical studies the parameters that have been used in clinical trials. Indeed, I believe that one of the reasons for the success of this journey has been the development of a solid scientific rationale. I was definitely fortunate to have worked with a team of incredibly skilled young scientists that shared with me the wish to help DMD patients and the perseverance to take on a challenge for over 20 years. Overall, discovering the MOA of HDACs has been a fantastic journey.”
“Although the evidence that HDACi could be used to treat DMD was strong and the rationale was very solid, it was hard to convince big pharma to invest on this treatment. There were many excellent HDACi in the market that could have been used, but after knocking on many doors, no one was willing to partner on this task.
Puri felt compelled by these results to keep building support to convince potential industry partners to develop clinical trials. After talking with many businesses, he found Italfarmaco, an Italian pharmaceutical company with an HDAC inhibitor called Givinostat.
“In the end, I had to knock on the door of Italfarmaco, which owned Givinostat — an HDACi that I had never tested in my previous preclincial studies. This was another coincidence since Dr. Christian Steinkulher, a friend and colleague, alerted me about the potential availability of a pan-HDACi called Givinostat from Italfarmaco.
“When I approached Italfarmaco, I immediately realized that they were not very well prepared to take on this type of research. They were not familiar with DMD, and they didn’t have any previous experience on muscular diseases. They also had no background on the epigenetic effects of Givinostat.
“Until that time, they used Givinostat mostly for its anti-inflammatory properties, rather than the epigenetic regulation of gene expression. However, Italfarmaco recognized some potential in this operation and decided to give to me the opportunity to perform preclinical studies with Givinostat for DMD.”
Clinical trials and regulatory approval
After additional preclinical studies to better understand how Givinostat worked, Italfarmaco informed Puri that the company was ready to develop a clinical trial.
The results of the phase II clinical trial were published in Neuromuscular Disorders in 2016. During the trial, the scientists looked at whether the composition of the muscle improved. Muscle biopsies taken from 19 subjects who were treated for more than a year demonstrated that the drug had caused an increase in muscle fiber area and a decrease in fat deposition, scar tissue and other hallmarks of DMD.
“It was remarkable to see that the same positive effects observed in mdx mice treated with HDACi were also observed in DMD boys treated with Givinostat,” noted Puri. “The reproducibility of the outcomes in preclinical studies and clinical trials emphasizes the importance of performing accurate preclinical studies and identifying reliable outcome measures, as we did with HDACi for DMD.”
The trial also helped the team determine what dose of Givinostat was safe and still effective to use in the pivotal phase III trial that was reviewed by the FDA before the agency granted approval.
Italfarmaco established ITF Therapeutics in January 2024 as a new division that is now responsible for marketing DUVYZAT™ in the U.S. ITF Therapeutics announced that the drug was available in the U.S. on July 25. Italfarmaco’s Marketing Authorization Application (MAA) for Givinostat to the European Medicine Agency (EMA) was validated in fall 2023. This means that the drug is eligible to be reviewed by the EMA. If approved, Givinostat can be made available throughout the European Union (EU).

Puri surfs a wave in San Diego. Surfing is his passion outside of the laboratory.
Paving the way forward
Puri is not resting on his laurels and the approval of the first non-steroidal drug to treat DMD.
“Right now, we have steroids, HDAC inhibitors and gene therapy. We are working on the idea that gene therapy and HDAC inhibitors without steroids can perfectly synergize.”

Lorenzo and his dog Mojo.
The researchers are also investigating ways to enhance the effect of HDAC inhibitors through the use of extracellular vesicles (EV) released from FAPs following the exposure to HDACi. EVs are small biological bubbles that the body uses to carry compounds between cells. They are non-immunogenic and therefore suitable for transplantation into dystrophic muscles.
Puri is also investigating whether there are treatment conditions (including dietary supplements or other synergistic molecules) that can improve the therapeutic efficacy of HDACi. The researchers want to test if HDAC inhibitors can treat other forms of muscular dystrophy beyond DMD.
As much as Puri is focused on the future and continuing to find new and better approaches to treat muscular dystrophy, he also appreciates the importance of this vital moment and how the FDA’s decision positions the field for even more innovation.
“While muscular dystrophy was formally described by scientists 40 years ago, it has been a part of the human story since the beginning. People have been chasing something that could help, and for so long there was nothing to offer. Right now, we are paving the way for even better treatments that will be found.”
More information on the development of HDACi as a treatment for DMD is available in the following manuscripts:
- Puri P.L., Avantaggiati M.L., Balsano C., San N., Graessmann A., Giordano A., and Levrero M. p300 is required for MyoD-dependant cell cycle arrest and muscle specific gene transcription. EMBO J. 16,369-383 (1997)
- Puri P.L., Sartorelli V., Yang X.J., Hamamori Y., Ogrizko , Howard B., Kedes L, Wang J.Y.J., Graessmann A., Nakatani Y., Levrero M. Differential roles of p300 and PCAF acetyltransferases in muscle differentiation. Mol. Cell 1, 35-45 (1997)
- Sartorelli V*., Puri P.L.* , Hamamori Y, Ogrizko V., Nakatani Y., Wang J.Y.J., Kedes L. Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol. Cell. 4, 725-734 (1999). *equal contribution
- Puri P.L., Iezzi, S., Stiegler P., Chen T.T., Shiltz L., Muscat G., Giordano A, Wang J.Y.J. and Sartorelli V. Class I histone deacetylases sequentially interact with MyoD and pRb during skeletal myogenesis. Mol Cell. 8, 885-897 (2001)
- Iezzi S., Cossu G., Nervi C. Sartorelli V., and Puri P.L. Stage-specific modulation of skeletal myogenesis by inhibitors of nuclear deacetylases Proc. Natl. Acad. Sci 99, 7757-7762 (2002)
- Iezzi S., Di Padova M., Serra C., Caretti G., Simone C., Maklan E., Zhao P., Hoffman E., Puri P.L. and Sartorelli V. Deacetylase Inhibitors Increase Muscle cell Size by Promoting Myoblast Recruitment and Fusion Through Induction of Follistatin. Dev. Cell. 5:673-84. (2004).
- Minetti G. C., Colussi c., Adami R., Serra C., Mozzetta C., Parente V., Illi B., Fortuni S., Straino S., Gallinari P., Steinkhuler C., Capogrossi M., Sartorelli V., Bottinelli R., Gaetano C., Puri P.L. Functional and morphological recovery of dystrophic muscles in mice treated with deacetylase inhibitors. Nature Medicine 12 (10): 1147-50 (2006)
- Colussi C., Mozzetta C., Gurtner A. , Illi B., Straino S., Ragone G., Pescatori M., Zaccagnini G., Rosati G., Minetti G., Martelli F., Ricci E., Piaggio G., Gallinari P., Steinkulher C., Capogrossi M.C., Puri P.L*, Carlo Gaetano*. A Common Epigenetic Mechanism Underlies Nitric Oxide Donors and Histone Deacetylase Inhibitors Effect in Duchenne Muscular Dystrophy. Proc. Natl. Acad. Sci 105, 19183-7 (2008) *Corresponding authors. PMCID:PMC2614736.
- Mozzetta C., Consalvi S., Saccone V., Tierney M., Diamantini A., Mitchel K.J., Marazzi G., Borsellino G., Battistini L., Sassoon D., Sacco A., Puri P.L. Fibroadipogenic progenitors mediate the ability of HDAC inhibitors to promote regeneration in dystrophic muscles of young, but not old mdx mice. EMBO Mol. Med. (2013), Apr;5(4):626-39 doi: 10.1002/emmm.201202096. [Epub ahead of print]. PMC Journal In Process.
- Consalvi S, Mozzetta C, Bettica P, Germani M, Fiorentini F, Del Bene F, Rocchetti M, Leoni F, Mascagni P, Puri P.L., Saccone V. Preclinical studies in the mdx mouse model of Duchenne Muscular Dystrophy with the Histone Deacetylase inhibitor Givinostat. Mol Med. 2013 Mar 27. doi: 10.2119/molmed.2013.00011. [Epub ahead of print]. PMCID: PMC3667212.
- Saccone V, Consalvi S, Giordani L, Mozzetta C, Barozzi I, Sandoná M, Ryan T, Rojas-Muñoz A, Madaro L, Fasanaro P, Borsellino G, De Bardi M, Frigè G, Termanini A, Sun X, Rossant J, Bruneau BG, Mercola M, Minucci S, Puri P.L. HDAC-regulated myomiRs control BAF60 variant exchange and direct the functional phenotype of fibro-adipogenic progenitors in dystrophic muscles. Genes & Development. 2014 Apr 15;28(8):841-57. doi: 10.1101/gad.234468.113. Epub 2014 Mar 28.
- Bettica P, Petrini S, D’Oria V, D’Amico A, Catteruccia M, Pane M, Sivo S, Magri F, Brajkovic S, Messina S, Vita GL, Gatti B, Moggio M, Puri P.L., Rocchetti M, De Nicolao G, Vita G, Comi GP, Bertini E, Mercuri E. Histological effects of givinostat in boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2016 Jul 11. pii: S0960-8966(16)30069-4. doi: 10.1016/j.nmd.2016.07.002. [Epub ahead of print]
- Sandonà M, Consalvi S, Tucciarone L, De Bardi M, Scimeca M, Angelini DF, Buffa V, D’Amico A, Bertini ES, Cazzaniga S, Bettica P, Bouché M, Bongiovanni A, Puri P.L., Saccone V. HDAC inhibitors tune miRNAs in extracellular vesicles of dystrophic muscle-resident mesenchymal cells. EMBO Rep. 2020 Sep 3;21(9):e50863. doi: 10.15252/embr.202050863. Epub 2020 Aug 5. PMID: 32754983
- Consalvi S, Tucciarone L, Macrì E, De Bardi M, Picozza M, Salvatori I, Renzini A, Valente S, Mai A, Moresi V, Puri P.L.. Determinants of epigenetic resistance to HDAC inhibitors in dystrophic fibro-adipogenic progenitors. EMBO Rep. 2022 Jun 7;23(6):e54721. doi: 10.15252/embr.202254721. Epub 2022 Apr 4. PMID: 35383427
- Mozzetta C, Sartorelli V, Steinkuhler C, Puri P.L.. HDAC inhibitors as pharmacological treatment for Duchenne muscular dystrophy: a discovery journey from bench to patients. Trends Mol Med. 2024 Mar;30(3):278-294. doi: 10.1016/j.molmed.2024.01.007. Epub 2024 Feb 26. PMID: 38408879.