Two scientific meetings in late March brought together researchers studying aging and its implications for disease
Scientists from San Diego and across the United States gathered March 26-27, 2025, to discuss the latest advancements in aging research. The NIH-funded San Diego Nathan Shock Center, a collaboration among the Salk Institute for Biological Studies, Sanford Burnham Prebys and the University of California San Diego, opened the two scientific meetings with its 2025 symposium on Wednesday, March 26, at the Salk Institute in the Conrad T. Prebys Auditorium in La Jolla.
The event focused on the center’s primary research area, “The Heterogeneity of Aging.” Just as people and organisms age at different rates, scientists have demonstrated that tissues also age at their own speeds – even some cells within tissues age at a unique pace. This phenomenon, known as heterogeneity of aging, is of great interest to researchers as it may hold clues for how to develop interventions that enable people to lead healthier lives as they age.
The San Diego Nathan Shock Center Symposium convened 193 in-person attendees and another 113 virtual participants over Zoom.
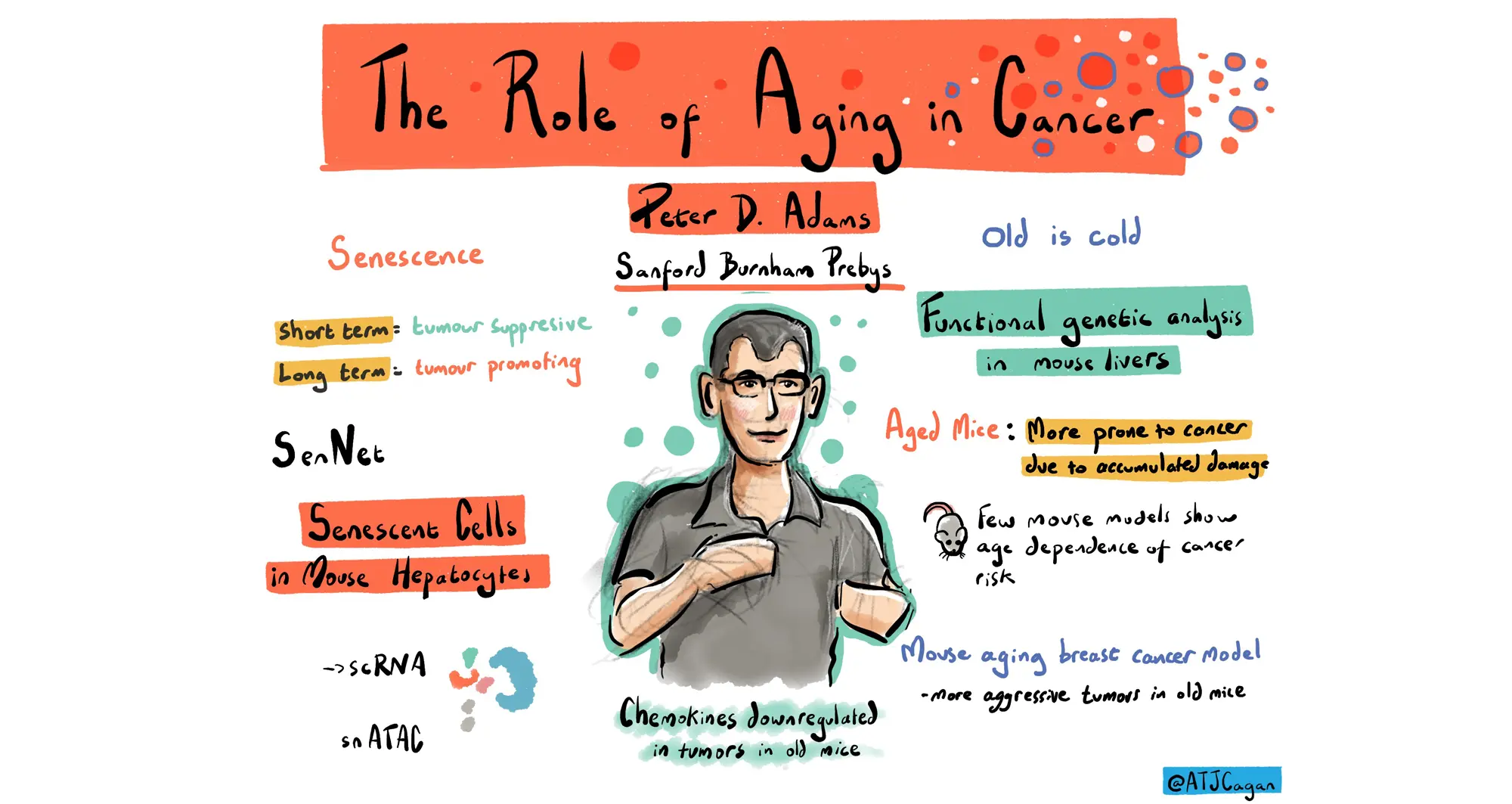
Shanshan Yin, PhD, a postdoctoral associate in the lab of Peter D. Adams, PhD, director and professor in the Cancer Genome and Epigenetics Program at Sanford Burnham Prebys, presented an update on her research regarding breast cancer and aging. She discussed results from investigating changes in gene expression and immune system activity in breast cancer tumors as mice age, leading to increased cancer incidence. Yin was awarded a San Diego Nathan Shock Center pilot grant in 2023.
The 8th annual La Jolla Aging Meeting was held on Thursday, March 27, also in Salk’s Conrad T. Prebys Auditorium. The event brought together 257 in-person attendees and featured mostly short talks from San Diego-based postdoctoral fellows and students researching the biology of aging.
Kelly Yichen Li, PhD, a postdoctoral associate in the lab of Kevin Yip, PhD, interim director of the Center for Data Sciences and professor in the Cancer Genome and Epigenetics Program, delivered a talk regarding her work on zombie-like senescent cells that persist but no longer divide like most normal cells. Li discussed her work exploring cell types in samples of liver tissue. She discovered differences in cell composition and gene expression based on the age of the samples. Li and her collaborators continue to work on methods to identify senescent cells in tissue samples, which would accelerate research in the field.
Tatiana Moreno, a graduate student in the lab of Caroline Kumsta, PhD, associate dean of Student Affairs in the Graduate School of Biomedical Sciences and assistant professor in the Center for Cardiovascular and Muscular Diseases, detailed her studies regarding aging and the body’s cellular recycling system, a process called autophagy. Moreno discussed her findings measuring autophagy in blood samples drawn from human volunteers of various ages, including results regarding the effects of a 12-week exercise program.
Rouven Arnold, PhD, a postdoctoral associate in the Adams lab at Sanford Burnham Prebys, presented his work seeking to better understand how aging can lead to a loss of the unique cellular identity that allows cells to carry out specialized functions in different organs. Arnold focused on the HIRA protein, one of the histone chaperones responsible for helping to build spools out of histones used to hold DNA like a thread. Following studies of HIRA’s role in the aging liver, his results suggest that HIRA plays a protective role to preserve liver cell identity and promote healthy aging in the liver.
Alessandra Sacco, PhD, professor and director of the Development, Aging and Regeneration Program in the Center for Cardiovascular and Muscular Diseases at Sanford Burnham Prebys, and dean of the institute’s Graduate School of Biomedical Sciences, was a cohost for both events. Adams was a cohost for the La Jolla Aging Meeting.
About the San Diego Nathan Shock Center
The San Diego Nathan Shock Center (SD-NSC), led by Gerald Shadel, PhD, Audrey Geisel Chair in Biomedical Science and professor in the Molecular and Cell Biology Laboratory at the Salk Institute, was established in the fall of 2020 with the overall goal of understanding the heterogeneity of aging in order to allow development of personalized interventions to increase the number of years of healthy life.
To this end, the center provides three novel scientific Research Resource Cores to develop new human cell models of aging and enable the integrated analysis of molecular, cellular and tissue heterogeneity. The SD-NSC also supports and advocates basic biology of aging research in general through the development, training and mentoring activities of a Research Development Core and robust outreach efforts. All of these activities are accomplished via a consortium of three premier research institutions on the La Jolla Research Mesa: the Salk Institute for Biological Studies, Sanford Burnham Prebys and the University of California San Diego.
Alessandra Sacco serves as director of the SD-NSC Research Development Core and Peter Adams serves as co-director of the SD-NSC Heterogeneity of Aging Core.