How a one-of-a-kind kid and his family stay connected during the pandemic
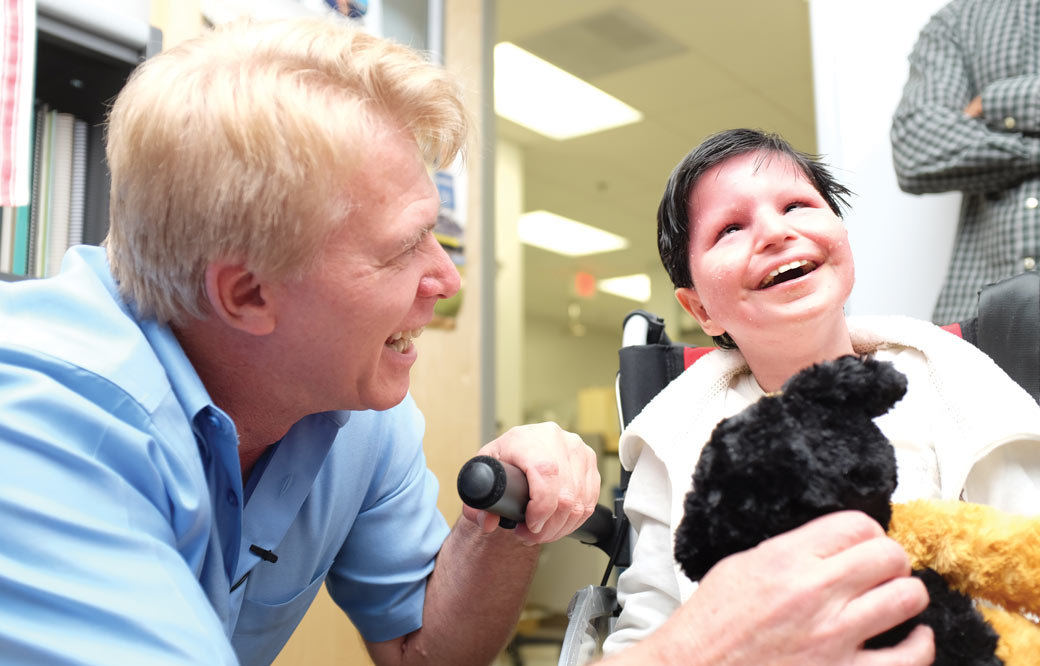
Thirteen-year-old Damian Omler is the only person in the world with his rare genetic mutation, which presents him and his parents (Donnie and Gracie) and 11-year-old brother, DJ, with major challenges every day. Damian’s condition—a congenital disorder of glycosylation, or CDG—causes him to have seizures, and requires him to have help with routine tasks such as using the restroom and dressing. And, he must use a wheelchair for mobility.
Despite these obstacles, Damian lives a rich, fulfilling life. But protecting his health during the COVID-19 pandemic threw a major wrench into the Omlers’ routine.
“In the early days of the pandemic, we didn’t know what kind of effect COVID would have on Damian, so we had to take a lot of precautions, including not seeing a lot of family and friends, which was very isolating,” says Donnie.
“Damian is also very sociable—we call him the hot potato because he just goes from person to person, so the pandemic was hard for him in that way as well,” adds Gracie. “We were so glad when we were finally able to get our family vaccinated so we could be more a part of the community.”
Staying at home had its ups and downs for the Omlers
Although most of us can relate to the isolation of the pandemic, there are unique challenges that come with being a family living with a rare disease during this time.
“Appointments were so much more difficult for Damian over Zoom,” says Gracie. “I had to help him through his physical therapy, and I was nervous that I might be doing it wrong or even hurting him.”
Despite these complications to Damian’s care, there were some unexpected silver linings to spending more time at home.
“Damian does choir and dance for his electives at school,” says Gracie. “I love that with remote learning I was able to interact with him and the class and learn the dances with him.”
“She definitely got a lot of accolades from the teachers for being one of the parents who participates,” adds Donnie, jovially.
Returning to Sanford Burnham Prebys’ Rare Disease Day
The Omlers are longtime friends of Sanford Burnham Prebys. They first visited the Institute in 2012, when Damian was 5. Before then, they’d been struggling to find a diagnosis for their son, who’d been missing developmental milestones since he was born.
With the help of Institute professor Hudson Freeze, PhD, who has dedicated his career to CDG research, doctors were finally able to diagnose Damian’s specific case in 2015.
“After the diagnosis, we sat and smiled for a long time,” says Donnie. “Just knowing was such a relief.”
Since 2016, the Omlers have also been regular participants in the Institute’s Rare Disease Symposiums, which help patients, researchers and clinicians from around the world connect in order to support one another and learn about the latest advances in rare disease research.
The most recent Rare Disease Day the Omlers attended was in 2020, just before the pandemic took hold. And although the event didn’t take place last year, this year it’s back stronger than ever. And the Omlers can’t wait to be back too.
“Meetings like this bring us hope and help us raise awareness for CDG,” says Donnie. “That gives us a sense of purpose each and every time we go. And we won’t stop, even 20 years from now.”
The 2022 Rare Disease Day Symposium & CDG/NGLY1 Family Conference will take place February 25–27 at the Dana Hotel on Mission Bay in San Diego. Scientific sessions will be held on the 25th and 26th, and the Family Conference will take place on the 27th.
And if you see a young man acting like a social “hot potato” on the 27th, that’s Damian. He’ll probably say hi to you.